Healthcare professionals handling chemotherapy for preparation and administration of HMPs are likely to be exposed to potential contamination risks associated with cytotoxic drugs. These risks can lead to health implications, ranging from minor issues like headaches, nausea, vomiting etc., to more serious conditions such as cancer and reproductive issues.
With the raising prevalence of cancer cases, healthcare workers’ exposure to these HMPs is also increasing, putting their health at risk.
In this article, our primary focus will be on the safe administration of cytotoxic drugs. We will examine the specific risks faced by healthcare workers in this context using evidence in literature, and then introduce Closed Safety Systems for Administration (CSSAs) as an important way to differentiate between CSTDs, which are devices dedicated to preparation commonly used in Pharmacy compounding units, and CSSAs, devices designed specifically for administration of chemotherapy by Nurses.
Both of these devices share a common goal: reduce exposure from Pharmacy Technicians when preparing chemotherapies and prevent contamination risk for Nurses administering treatment to patients, through perfusion via an IV line, a syringe, an elastomeric pump or even a cassette.
WHAT ARE HMPS?
According to the Guidance for the safe management of hazardous medicinal products at work1, HMP is defined as a medicinal product that contains one or more substances that meet the criteria for the CMR (Carcinogenic, Mutagenic, Reprotoxic) classification:

HMPs include cytotoxic, cytostatic, and antineoplastic medicinal products that are highly toxic to cells (including healthy cells), with many being CMR. These medicinal products are used in a variety of clinical and laboratory settings for the treatment of cancer and other medical conditions.
There is an ongoing debate about whether monoclonal antibodies (MABs) are HMPs. Monoclonal antibodies are used for the treatment of a wide range of conditions in haematology and oncology, graft rejection, inflammatory or auto-immune diseases. However, some conjugated monoclonal antibodies and one monoclonal antibody that is not conjugated to a cytotoxin have been included in the National Institute for Occupational Safety and Health (NIOSH) list of Antineoplastic and other Hazardous Drugs in Healthcare Settings.
The risks of occupational exposure to HMPs
HD contamination has been documented over the past four decades, starting in 1970. Scientific evidence highlights that exposure to cytotoxic drugs can result in both immediate and lasting health concerns for healthcare professionals.
Acute effects reported among healthcare workers encompass symptoms like nausea, headaches, rashes, diarrhea, coughing, hair thinning, and hair loss2,3.
Additionally, research has consistently demonstrated that healthcare workers may suffer from enduring chronic health repercussions, including elevated rates of foetal loss, congenital malformations, infertility, and cancer4. Up to now, there are more than 100 clinical papers reporting adverse events and contamination risks on nurses exposed to occupational exposure. The first convincing evidence was reported by Falck et al5 in 1979, when contamination was found in urine from nurses administering chemotherapy to patient. Another example is a study conducted by Skov et al. in 1992 that revealed an increased risk of leukaemia among oncology nurses recorded in the Danish cancer registry6.
Also, in 2012, the EU Commission evaluated that up to 107.000 deaths from cancer could be attributed to professional exposure to carcinogenic substances. Still in Europe, it has been reported in 2017 that professional exposure to these substances led to more than 17.000 miscarriages and more than 10.000 reproductive outcomes and were responsible for 2.220 new cases of leukaemia among healthcare professionals.7

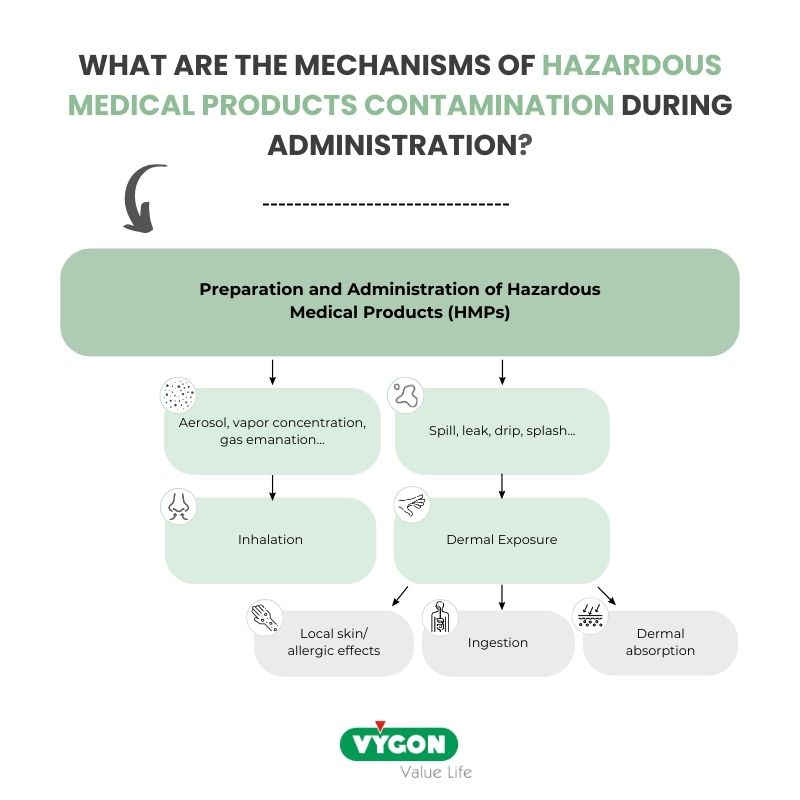
The mechanisms of HMPs contamination during administration
According to the article titled “Controlling Occupational Exposure to Hazardous Drugs” by the Occupational Safety and Health Administration (OSHA)8, contamination of healthcare workers with cytotoxic drugs can occur through different pathways:
- Absorption: Exposure can occur through absorption when liquid HDs leak or spill during tasks like “connecting or disconnecting tubing or syringes,” “priming air from infusion sets or syringes”, or “accidental disconnections in drug delivery systems”.
- Inhalation: Inhalation is a potential route of exposure, with HD dust and aerosols being generated during activities such as “inserting or removing tubing from IV containers”, “expelling air from syringes”, or “clipping/crushing needles or syringes”.
- Dermal exposure: it is a key exposure route. Exposure during HMPs administration will occur mainly by dermal exposure, due to the contact with contaminated workplace surfaces.
- Exposure by ingestion will be negligible as drinking and eating must be forbidden during the handling of HMPs. However, hand-to-mouth contact may still result in exposure by ingestion.
Protecting healthcare workers using CSTDs
In recent years, the healthcare industry has witnessed the emergence of innovative devices aimed at significantly enhancing the safety of healthcare workers involved in the preparation, administration, and handling of cytotoxic drugs.
The National Institute for Occupational Safety and Health has introduced for the 1st time in 2004 the definition of a Closed-system transfer devices (CSTD) as a “a drug transfer device that mechanically prohibits the transfer of environmental contaminants into the system and the escape of the hazardous drug or vapor concentrations outside the system.”
Research has underscored the effectiveness of CSTDs in mitigating the risks associated with chemotherapy exposure. In a multicentre evaluation conducted by Bartel et al in 2018, the authors evaluated the effectiveness of CSTDs in reducing surface contamination during compounding and simulated administration of antineoplastic hazardous drugs. Their findings revealed that in administration areas alone, the rate of preexisting contamination was 78%, when in comparison, a contamination rate of 2.6% was found when using CSTDs.
CSSAs as a device for administration
CSTDs primarily find use in preparing cytotoxic drugs and are not ideally designed for administration. This limitation, as underscored in a study10 by Seth Eisenberg published in the British Journal of Nursing, is rooted in their historical emphasis on pharmacists engaged in cytotoxic drug preparation, neglecting nurses who administer these products at the patient’s bedside.
Furthermore, a critical concern lies in the absence of a dedicated training program on possible risks, as well as a limited involvement of nurses in choosing protective medical devices. These factors raise significant concerns about the safety of healthcare workers at the bedside. Therefore, proper education and training are essential for minimizing contamination risks when administering hazardous drugs. CytoPrevent, a group of experts dedicated to increasing protection for healthcare workers during handling, preparing, or administering cytotoxic drugs, has introduced the term ‘Closed Safety System for Administration’ (CSSA)10. According to CytoPrevent, a CSSA11 is a Luer format product that connects the distal end of IV lines, including elastomeric and electronic pump tubing, or syringes to a needleless connector, effectively eliminating exposure.
Distinguishing CSSAs from CSTDs is crucial because it allows a clear understanding of both devices benefits for their specific usage in order to focus on educating healthcare professionals specifically about hazardous drug contamination during administration. Additionally, Luer-to-Luer CSSAs are specifically designed for administration, while membrane-to-membrane CSTDs are more dedicated to the preparation of drugs and require a Luer adapter to comply with SOP (Standard of Procedure) for use in administration. The latter results in extra components and increased costs, which can pose an obstacle in the general use of CSSAs10. Thus, by differentiating between CSTDs and CSSAs and promoting devices specifically designed for a safer administration of chemotherapy, the introduction of CSSAs as compared to CSTDs can drive positive changes in nursing practice.
Conclusion
The underestimation of exposure risks affecting nurses during administration requires greater attention from all the stakeholders. While measures are taken to safeguard pharmacists during compounding process, there is an insufficient focus on ensuring nurses’ safety in Day care units or in Hospitalization units. The limited historical use of CSSAs in administration and the lack of education and awareness about the associated risks, hinder their adoption in administration. Promoting the use of CSSA’s sing proper terminology and education can transform practices in the field and safeguard the health of healthcare professionals.
BIBLIOGRAPHY
- European Commission – Guidance for the safe management of hazardous medicinal products at work – 04/2023
- ONS, Safe handling of hazardous drugs, Second Edition – https://www.ons.org/sites/default/files/publication_pdfs/2_Safe%20Handling_pages_CHAPTER_1.pdf
- Jeffrey Lombardo and Christine Roussel, Highlighting the risk of occupational exposure to hazardous drugs in the healthcare setting, November 2018 – https://www.pharmacytimes.com/view/highlighting-the-risk-of-occupational-exposure-to-hazardous-drugs-in-the-health-care-setting-
- CDC, Preventing Occupational Exposures to antineoplastic and other hazardous drugs in healthcare settings, September 2004 – https://www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf
- Falck et al. Mutagenicity in urine of nurses handling cytostatic drugs Lancet Journal 1979 – https://pubmed.ncbi.nlm.nih.gov/87722/
- Skov et al. 1992, Leukaemia and reproductive outcome among nurses handling antineoplastic drugs – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1061216/
- The Parliament Magazine, Preventing the exposure of nurses and health professionals to hazardous drugs, June 2017 – https://www.theparliamentmagazine.eu/articles/partner_article/efn/preventing-exposure-nursesand-health-professionals-hazardous-drugs
- Occupation Safety and Health Administration, Controlling occupational exposure to hazardous drugs – https://www.osha.gov/hazardous-drugs/controlling-occex
- Bartel et al, Am J Health-Syst Pharm, Multicentre evaluation of a new closed system drug-transfer device in reducing surface contamination by antineoplastic hazardous drugs, 2018, Volume 75 – https://pubmed.ncbi.nlm.nih.gov/29339374/
- Seth Einserberg,Closed safety system for administration (CSSA): proposal for a new cytotoxic chemotherapy acronym, May 2022 – https://pubmed.ncbi.nlm.nih.gov/35648666/
- Seth Einsenberg, Hazardous drug safety: Clarifying the confusion about closed systems – https://cytoprevent.eu/wp-content/uploads/sites/10/2022/09/Hazardous-Drugs-Safety-Clarifying-the-confusion-about-closed-systems.pdf