Emergency and critical care teams face a recurring dilemma in early septic shock: How much fluid is enough—and when should we start vasopressors? Prolonged hypotension drives organ injury and mortality, but both fluid overload and delayed vasopressors can harm. The Surviving Sepsis Campaign (SSC) historically suggests 30 mL/kg initial crystalloid, yet that “one‑size‑fits‑all” dose is a weak recommendation and increasingly questioned in favor of individualized resuscitation.1
WHAT THE PHYSIOLOGY AND TRIALS TELL US
Septic shock is predominantly vasoplegic—systemic vasodilation and vascular leak impair perfusion. Mortality remains high (often reported up to ~60% in some settings), and the degree and duration of hypotension are major outcome determinants, making rapid restoration of MAP ≥65 mmHg a core goal (with higher targets for some chronic hypertensives).1
Large early fluid loads have long been used to raise MAP, but dynamic monitoring shows not all patients are fluid responsive; unnecessary fluid increases positive balance, delays organ recovery, prolongs ICU stay, and is associated with higher mortality. Emerging evidence favors earlier norepinephrine (NE) to treat vasodilation, often within the first hour, to correct pressure faster, recruit perfusion, and avoid fluid overload.1
THE PROBLEM WITH DOING “FLUIDS FIRST” EVERY TIME
- Over-resuscitation risks: Positive fluid balance correlates with higher mortality and longer stays; the SOAP analysis and other cohorts link fluid overload to worse outcomes.1
- Variable responsiveness: Static signs (HR, BP) are poor guides; dynamic indices and bedside ultrasound outperform them for predicting fluid responsiveness.1
- Guideline nuance: SSC’s 30 mL/kg is not a must for all—especially in cardiac or renal dysfunction; personalization is advised.1
WHAT CLOVERS ADDS—AND WHAT IT DOESN’T
The CLOVERS randomized trial compared restrictive (vasopressors earlier, less fluid) versus liberal (more fluid before vasopressors) strategies for the first 24 hours after patients had already received 1–3 L. Mortality before discharge home by day 90 was similar (14.0% vs. 14.9%; P = 0.61). The restrictive arm used significantly less fluid, earlier and longer vasopressors, with no safety signal difference overall (including very low rates of peripheral vasopressor complications). Bottom line: Both approaches yielded comparable outcomes in this context, underscoring the need to tailor strategy to patient physiology rather than defaulting to volume alone.2
Important nuance: CLOVERS does not endorse flooding patients; it shows that after early initial fluids, prioritizing vasopressors or additional fluids for 24 hours produced similar mortality—while confirming the feasibility and safety of earlier vasopressor use, including peripheral starts when central access is not yet available.2
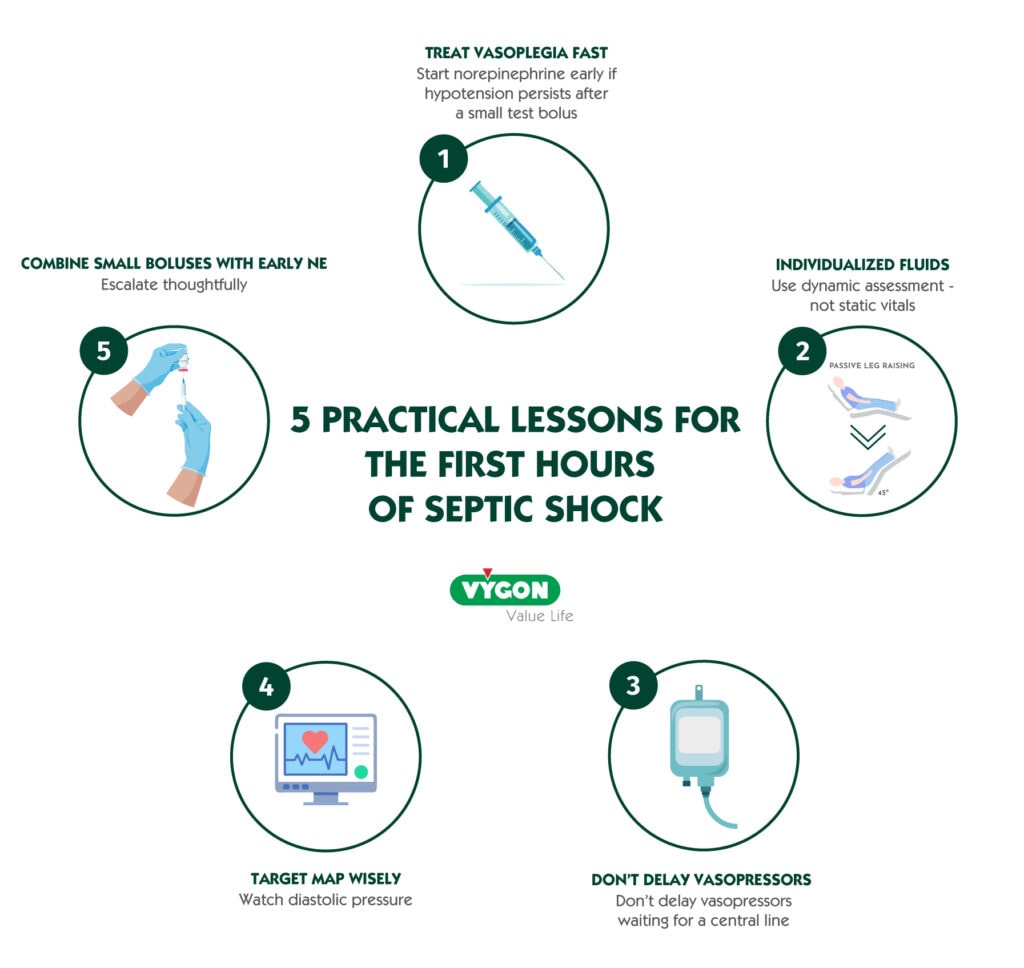
5 PRACTICAL LESSONS FOR THE FIRST HOURS OF SEPTIC SHOCK
1) Treat vasoplegia fast—start norepinephrine early if hypotension persists after a small test bolus
If MAP remains <65 mmHg after ~5–10 mL/kg or a 300–500 mL bolus, consider early norepinephrine rather than repeating large fluid boluses. Early NE can raise MAP quicker, decrease preload dependency by mobilizing stressed volume, and reduce fluid requirements—benefits linked to better hemodynamics and fewer complications in observational data and trials like CENSER.1
2) Individualize fluids using dynamic assessment—not static vitals
Use passive leg raise, stroke volume change, or point‑of‑care ultrasound (e.g., LV filling, IVC dynamics) to decide if a patient will actually increase cardiac output with more fluid. This reduces the risk of overload without compromising perfusion.1
3) Don’t delay vasopressors waiting for a central line
Initiate NE peripherally (appropriately sized IV, vigilant monitoring) when central access is not yet secured. Recent clinical experience shows very low rates of self‑limited extravasation when done carefully—supporting earlier correction of hypotension.1,2
4) Target MAP wisely—and watch diastolic pressure
Aim for ≥65 mmHg in most, but consider ~80 mmHg in chronic hypertensives or special conditions (e.g., raised ICP, high CVP). A diastolic arterial pressure <60 mmHg or a high Diastolic Shock Index (HR/DBP >2.2) may flag profound vasoplegia and the need for prompt vasopressors.1
5) Combine small, mindful boluses with early NE; escalate thoughtfully
In fluid‑responsive patients, small boluses alongside NE can improve mean systemic filling pressure without the harms of large volume loads; if MAP remains inadequate on NE, vasopressin is a reasonable add‑on before escalating NE dose further (evidence for mortality benefit remains mixed).1
PUTTING IT TOGETHER: A SIMPLE BEDSIDE ALGORITHM
- Initial actions (minutes 0–15):
- Obtain IV access, cultures, antibiotics, lactate, source control as indicated.
- Give an initial 300–500 mL crystalloid bolus while you set up dynamic assessment.1
- Assess response (minutes 15–30):
- If fluid responsive, consider another small bolus; if not responsive or signs of overload, stop fluids.
- If MAP <65 mmHg, start NE (peripheral if needed).1
- Stabilize macro‑perfusion (first 60 minutes):
- Titrate NE to MAP target (often ≥65 mmHg; higher in selected patients).
- Reassess for fluid responsiveness and signs of congestion after any bolus.1
- First 6–24 hours:
- Continue personalized fluids only when responsive; avoid automatic “30 mL/kg.”
- Add vasopressin if NE requirements rise and perfusion is still inadequate.
- Remember: In CLOVERS, a restrictive strategy (more vasopressors, less fluid) did not reduce 90‑day mortality vs. a liberal strategy after 1–3 L—use clinical judgment and physiology to steer the course.1, 2
KEY TAKEAWAYS
- Avoid fluid reflexes. Use dynamic tests to decide who benefits from more volume.1
- Correct vasoplegia early. Starting norepinephrine sooner can restore MAP faster and curb fluid exposure.1
- Safety first. Peripheral NE is acceptable as a bridge; monitor the site closely.1,2
- No single recipe. CLOVERS shows both strategies can be safe after early fluids; individualization is the winning principle.2
Modern septic shock resuscitation is not “fluids first for everyone.” The signal across physiology, trials, and practice is to personalize fluid therapy and adopt early vasopressors when hypotension persists after a small, diagnostic bolus—reducing the risk of fluid overload while restoring perfusion rapidly. CLOVERS reminds us that after an early liter or two, both fluid‑forward and vasopressor‑forward pathways can achieve similar outcomes—so let patient phenotype and responsiveness guide your next move.1, 2
BIBLIOGRAPHY
- Sanchez CE, Pinsky MR, Sinha S, et al. Fluids and Early Vasopressors in the Management of Septic Shock: Do We Have the Right Answers Yet? J Crit Care Med. 2023;9(3):138‑147. doi:10.2478/jccm-2023-0022.
- Shapiro NI, Douglas IS, Brower RG, et al. Early Restrictive or Liberal Fluid Management for Sepsis‑Induced Hypotension (CLOVERS). N Engl J Med. 2023;388(6):499‑510. (Author manuscript, HHS Public Access). doi:10.1056/NEJMoa2212663.