Context
Patrick Bouvier Kennedy, son of John F. Kennedy; President of the United States, was born on August 7th1963, five weeks before his due date. After his birth he was transferred to one of the best children’s hospitals, Boston Children’s Hospital, and treated by renowned specialists, including the use of a hyperbaric chamber. Despite all their efforts, Patrick barely survived 39 hours and died on August 9th as a result of Hyaline Membrane Disease (now known as respiratory distress syndrome). After this tragedy, John F. Kennedy released funds to advance research on newborn babies.
To give you an idea of the progress made in neonatal survival since then: Patrick was delivered at 34-weeks’ gestation, nowadays a baby born between 32- and 34-weeks’ gestation has a chance of survival of almost 99%. (1) This little story allows us to assess the enormous progress that neonatology has made in a few decades, with a remarkable reduction in the limits of viability and management of the neonatal and premature patient.
But… What is Respiratory Distress Syndrome?
Among the pathologies that have evolved in its assessment and management, has been the respiratory distress syndrome (RDS) of the neonate, known as a primary disorder associated with prematurity, specifically pulmonary immaturity and, to a lesser extent, of the airways.
The main cause of RDS is an insufficient amount of pulmonary surfactant. The most common etiologic factor is premature birth. The manifestations of the disease are due to the resulting alveolar atelectasis, edema and cellular injury. As a result, serum proteins that inhibit surfactant function leak into the alveolus. The higher water content, immature pulmonary fluid clearance mechanisms, lack of alveolocapillary apposition, and reduced gas exchange surface characteristic of the immature lung also contribute to the disease. The ability to perform prenatal diagnosis to identify infants at risk, prevention of the disease through prenatal corticosteroid administration, improved perinatal and neonatal care, and advances in respiratory support and surfactant replacement therapy have reduced the mortality of RDS.
Know the keys to managing newborns with RDS
As mentioned above, the greatest knowledge and progress in newborns management is that there is now an improvement in treatment and care, so we must not forget the keys to the management of neonates with RDS, which are:
- Preventing hypoxemia and acidosis (allowing normal tissue metabolism, optimizing surfactant production and avoiding the occurrence of right-to-left shunts)
- Optimize fluid control (avoid hypovolemia, shock and edema, especially pulmonary edema)
- Reduce metabolic demands
- Avoid worsening of atelectasis and pulmonary edema; (v) minimize oxidative lung injury
- Reduce mechanical ventilation-induced lung injury.
Despite all of the above, RDS remains an important cause of neonatal mortality and morbidity, especially in the most premature newborns (2).
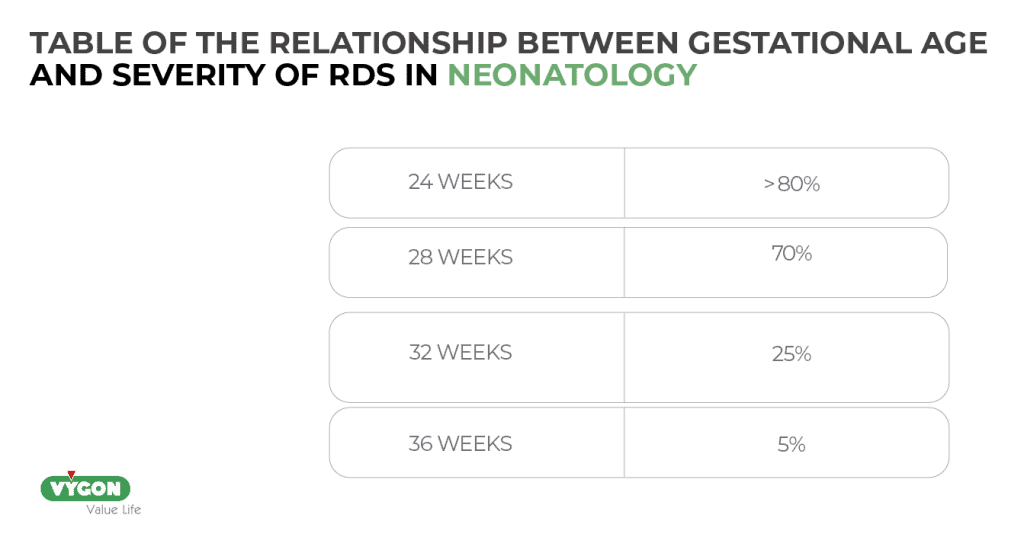
As mentioned above, one of the most important (if not the most important) triggering factors in the etiology of RDS is prematurity. In general, the incidence and severity of RDS is inversely related to gestational age (the lower the gestational age, the higher the risk of RDS) (3):
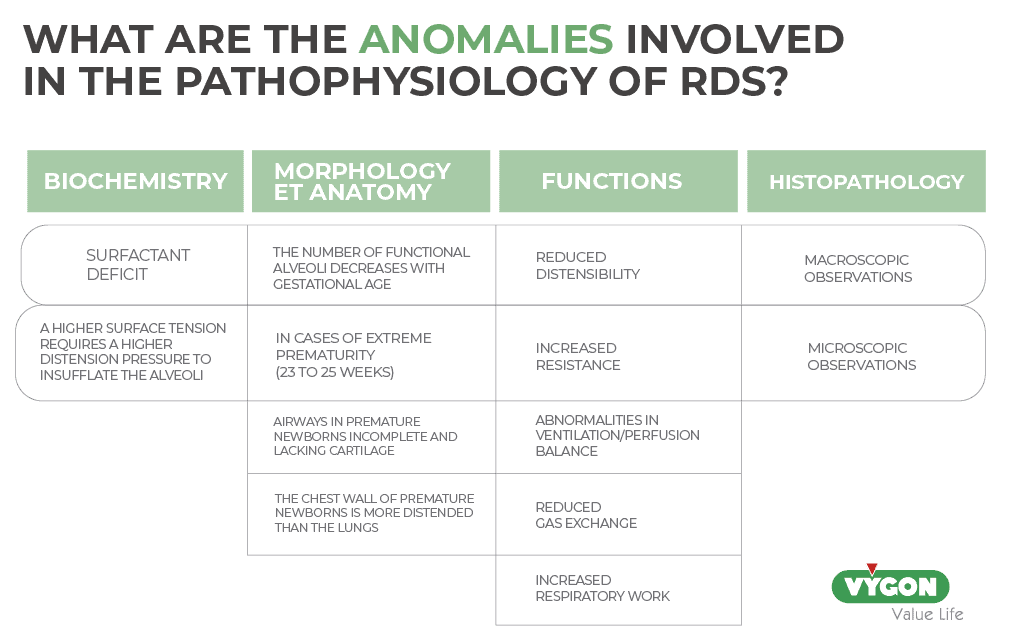
What pathologies are covered by the pathophysiology of RDS?
To understand a pathology and to be able to correlate its manifestations and the origin of its bases, knowledge of the pathophysiology is essential, which for RDS includes: (3)
Biochemical abnormalities
a) The main feature is a surfactant deficiency, which leads to increased tension at the alveolar surface level and interferes with normal gas exchange.
b) According to Laplace’s law, the higher surface tension requires a higher distending pressure to insufflate the alveoli:

As the radius of the alveolus decreases (atelectasis) and its surface tension increases, the amount of pressure required to overcome these forces increases.
Morphologic and anatomic abnormalities
- The number of functional alveoli increases with gestational age. A premature baby will therefore have a smaller surface area for gas exchange.
- In case of extreme prematurity (23 to 25 weeks), the distance between the alveolus or terminal bronchiole and the nearest adjacent capillary increases, thus increasing the diffusion barrier, which interferes with the transport of oxygen from the lungs to the blood.
- The airways of the premature infant are incompletely formed and don’t have enough cartilage to remain patent. This can lead to collapse and increased airway resistance.
- The chest wall of the preterm infant is more distensible than the lungs, so it tends to collapse as the neonate attempts to increase intrathoracic negative pressure and increase work of breathing.
Functional abnormalities
- Decreased distensibility
- Increased resistance
- Abnormal ventilation/perfusion balance
- Decreased gas exchange
- Increased work of breathing
Histopathologic abnormalities
- Autopsies of neonates dying of RDS reveal an almost uniformly airless lung. Microscopic examination reveals diffuse atelectasis surrounding a few terminal bronchioles and greatly dilated alveolar ducts. A fibrinous eosinophilic (or hyaline) membrane containing cellular debris derived from blood and damaged epithelium lines these airspaces. This common postmortem finding established the original nomenclature of RDS as a hyaline membrane disease (4).
- Macroscopic findings:
- Decreased aeration.
- Stiff, rubbery, “liver-like” lungs.
- Decreased lung volumes.
- Decreased aeration.
- Microscopic findings:
- Airspaces filled with a cosinophilic exudate composed of proteinaceous material, with and without inflammatory cells.
- Airspaces edema.
- Alveolar collapse.
- Squamous metaplasia of the airway epithelium.
- Distention of the lymphatic vessels.
- Thickening of the pulmonary arterioles.
Although a definitive diagnosis of RDS requires pathologic or biochemical documentation of surfactant deficiency, clinicians often use a combination of clinical and radiographic features to diagnose RDS.
What are the clinical manifestations of RSD?
Clinical symptoms appear shortly after birth and include:
- Wheezing
- Tachypnea: the affected neonate breathes rapidly and attempts to compensate for small tidal volumes by increasing respiratory rate and minute ventilation to eliminate carbon dioxide.
- Nasal flaring: this increases the transverse airway of the nasal passages and decreases upper airway resistance.
- Whimpering: this is an attempt by the neonate to generate positive end-expiratory pressure (PEEP) by exhaling against a closed glottis. The aim is to maintain a certain alveolar volume (distention) so that the radius of the alveoli is larger, and less work is required to expand them.
- Retractions: The neonate uses accessory respiratory muscles, such as the intercostal muscles, to provide the increased pressure needed to insufflate the lungs.
- Cyanosis: Reflects decreased oxygenation, when there is more than 5g/dL of deoxygenated hemoglobin.
In severe cases of RDS, neonates may progress to respiratory failure requiring intubation and mechanical ventilation (4).
Litterature
- Ancel PY, Goffinet F; EPIPAGE-2 Writing Group; et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015.
- John P. Cloherty, Eric C. Eichenwald, Anne R. Hansen, Ann R. Stark. Manual de Neonatología. 7ª edición. España. Wolters Kluwer España, S.A., Lippincott Williams&Wilkins. 2012.
- Yadav S, Lee B, Kamity R. Neonatal Respiratory Distress Syndrome. 2023 Jul 25. StatPearls Publishing; 2024 Jan.
- Christopher McPherson, Jennifer A. Wambach. Neonatal Network. Vol 37. Nº 3. Prevention and Treatment of Respiratory Distress Syndrome in Preterm Neonates. Springer Publishing Company. 2018.







