The removal of totally implanted central venous access devices (TVAD), or Ports is associated with the risk of adverse events, among which some are insignificant and others more relevant or serious.
The technique of appropriate removal has only been described in a few manuals, but there is no standardisation of this technique. In this study review we will look at a recently standardised protocol known as SaRePo (Safe Removal of Ports), which consists of seven strategies to adopt during the removal of totally implanted venous access devices, in order to minimise the risk of adverse events.
These strategies include:
- Assessment of the patient’s medical history
- Pre-removal ultrasound
- Aseptic technique
- Local anaesthesia
- Catheter removal
- Reservoir removal from the subcutaneous pocket
- Incision closure
These devices may be located in various areas, such as:
- PICC-Port in the mid-arm
- FICC in the thigh
- Chest port due to its placement in the patient’s thorax
While the implantation technique is standardised through scientifically documented procedures, port removal is not as frequently addressed in scientific literature. Existing literature has focused on removal under conditions including:
- Completion of treatment
- When the device is no longer suitable
- Complications requiring removal
- Patient refusal to maintain the device
It is also noted that few publications discuss the complications involved in removing totally implanted venous access devices, and therefore standardised and strict approaches are often overlooked.
Some of the most common complications include excessive bleeding during removal, the silicone material of the catheter, prolonged duration causing tissue adhesions to the catheter chamber, or chronic inflammation due to extravasation. These complications create challenges for both healthcare professionals and patients during catheter removal.
However, some of these complications can be prevented or anticipated through proper evaluation of the patient’s history and appropriate removal protocols.
GAVeCeLT (Gli Accessi Venosi Centrali a Lungo Termine) has developed standardised vascular insertion protocols, with step-by-step strategies to improve safety and prevent complications in venous access procedures.(6)
In response to the published safe insertion procedures, a group of experts from GAVeCeLT developed a protocol focused on the port removal procedure under the name “SaRePo Protocol” (Safe Removal of Ports). This protocol consists of seven key recommendations, based on the best available evidence, to minimise complications during port removal in all patients, both adults and children.
SaRePo Protocol (Safe Removal of Ports)
The seven steps of the protocol:
- Patient’s medical history
- Preoperative ultrasound
- Aseptic technique
- Local anaesthesia and skin incision
- Catheter removal
- Reservoir removal
- Incision closure
1. Patient’s Medical History
The first step is to verify the correct reason for TVAD removal: end of treatment, complications requiring removal, need for a more suitable device, or patient decision.
Similar to recommendations for TVAD insertion, the patient’s coagulation status should be assessed. In cases of low platelet count and a clear indication for immediate removal, platelet transfusion should be considered shortly before the procedure.
Antithrombotic treatment or dual antiplatelet therapy, if present, should be discontinued according to GAVeCeLT consensus recommendations.
The patient’s medical history should also be reviewed to identify conditions that may be associated with difficult removal: prolonged device retention, possible pinching due to blind infraclavicular approach to the subclavian vein, high puncture of the internal jugular vein, silicone catheter, reservoir in an abnormal location. Preoperative evaluation should also include assessment of the skin over the reservoir to rule out local inflammation, discharge, or skin erosion.
Antibiotic use is only justified if the TVAD is being removed due to infection. The patient must be fully informed about the technical aspects of the procedure and sign an informed consent. Removal should preferably be performed in a dedicated procedure room: it is not recommended to perform it in an operating theatre or radiology room, as using these facilities would not increase safety but would reduce cost-effectiveness.
2. Preoperative Ultrasound
Ultrasound is useful for ruling out unrecognised complications, such as secondary malposition of the catheter tip or asymptomatic venous thrombosis.
Although not mandatory, preoperative ultrasound is cost-effective and feasible if equipment is available, helping to anticipate complications during port removal. If venous thrombosis is identified, antithrombotic treatment should be initiated and port removal delayed by at least 72 hours. Ultrasound can anticipate difficulties in catheter removal if there is a fibrous cuff or granuloma. X-ray and bubble test help detect fractures or structural damage due to pinching, preventing complications such as embolisation to the heart or lungs.
3. Aseptic Technique
Hand hygiene and skin antisepsis with 2% chlorhexidine in 70% isopropyl alcohol are recommended.
Use a non-sterile cap, non-sterile surgical mask, and sterile gloves. The use of a sterile surgical gown is not strictly necessary. Using all-inclusive procedure packs simplifies the procedure and reduces costs.
4. Local Anaesthesia and Skin Incision
Removal is best performed by reopening the previous surgical incision made to insert the reservoir into the subcutaneous pocket. The new incision is made along the previous scar, after appropriate local infiltration with local anaesthetics. In tunnelled ports, two incisions may be required. If there is exposure or inflammation, a rhomboid incision is recommended to remove pathological tissue and previous scar.
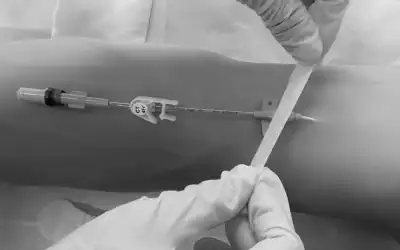
5. Catheter Removal
After freeing the catheter from the connective cuff which usually surrounds it in the subcutaneous tissue, it should be slowly removed from within the cuff.
The catheter must always be inspected after removal to rule out damage or breakage and to verify that it has been removed in its entirety.
If resistance to catheter removal is due to the fibrous sheath, the catheter should be interrupted near the connection with the reservoir and a metal guide inserted into the catheter. The catheter will then feel stiffer and be easier to release from the subcutaneous connective sheath.
6. Reservoir Removal
After removing the catheter, the reservoir is removed. Reservoir removal may be complicated by thick tissue capsule, deep location, distant incision, or if it is sutured to the muscle.
7. Incision Closure
Closure is performed with inverted dermal stitches, absorbable monofilament thread, and cyanoacrylate adhesive to effectively seal the epidermis. The incision is then covered with gauze and a transparent semi-permeable membrane, or with a transparent membrane only. After a few days, the dressing is removed.
Conclusion
Complications during TVAD removal are not common, but when they occur, they can be potentially significant and include bleeding, difficult or impossible catheter removal, catheter fragment embolisation into the circulation, infection, wound dehiscence, and seroma.
As suggested in the recent retrospective study by Su, Liu, Xie, et al., a thorough pre-removal evaluation and strict adherence to a well-defined removal procedure protocol can effectively anticipate removal difficulties and prevent complications.(5)
This SaRePo protocol: Safe Removal of Ports, developed by GAVeCeLT; includes seven steps that may be useful if used as a procedural checklist.
Preventing complications during port removal depends on adherence to current recommendations regarding proper insertion, maintenance, and safe removal.
Current recommendations (1,2,3,4) advise against blind infraclavicular puncture of the subclavian vein, “high” puncture of the internal jugular vein in the mid-neck, use of silicone catheters, and prolonged maintenance of TVADs that are no longer in use. Avoiding these four outdated and inappropriate strategies is likely to be associated with a significant reduction in the incidence of adverse events during port removal.
References
- Jahanzeb M, Wu CY, Lim H, et al. Consenso internacional de expertos sobre la selección y el manejo óptimos de dispositivos de acceso vascular central para pacientes con cáncer. J Vasc Access. Publicación electrónica previa a su impresión, 2 de diciembre de 2024. DOI:10.1177/11297298241300792.
- Nickel B, Gorski L, Kleidon T, et al. Estándares de práctica en terapia de infusión, 9.ª edición. J Infus Nurs 2024; 47(1S Suppl 1): S1–S285.
- Lamperti M, Biasucci DG, Disma N, et al. Guías de la Sociedad Europea de Anestesiología sobre el uso perioperatorio de acceso vascular guiado por ecografía (acceso vascular PERSEUS). Eur J Anaesthesiol 2020; 37(5): 344–376. Fe de erratas en: Eur J Anaesthesiol. Julio de 2020; 37(7):623.
- Annetta, M. G., Pinelli, F., Miluy, G. O., Scoppettuolo, G., & Pittiruti, M. (2025). The SaRePo protocol: A seven-step strategy to minimize complications potentially related to the removal of totally implanted central venous access devices. The Journal Of Vascular Access. https://doi.org/10.1177/11297298251333863
- “Su J, Liu L, Xie Y, et al. Complications associated with the removal of totally implantable venous access devices (TIVAD): a retrospective analysis of 4954 breast cancer patients in a single institution. BMC Surg 2024; 24(1): 324.
- Pittiruti M y Scoppettuolo G. Raccomandazioni GAVeCeLT, https://www.gavecelt.it/nuovo/sites/default/archivos/cargas/raccomandazioni-gavecelt-2024.pdf (2024, consultado enero de 2024).