The introduction of ENFit® connectors in neonatology units, with the aim of avoiding connection errors between systems intended for different clinical therapies, has generated much debate among experts in the field.
The new connector for enteral nutrition poses a number of challenges for patients in neonatology services. Along with dosing accuracy issues due to the risk of displacement of accumulated formula in the connector of the new syringe design, concerns have arisen in implementation related to cleaning procedures for the probes or extenders to which they are connected.
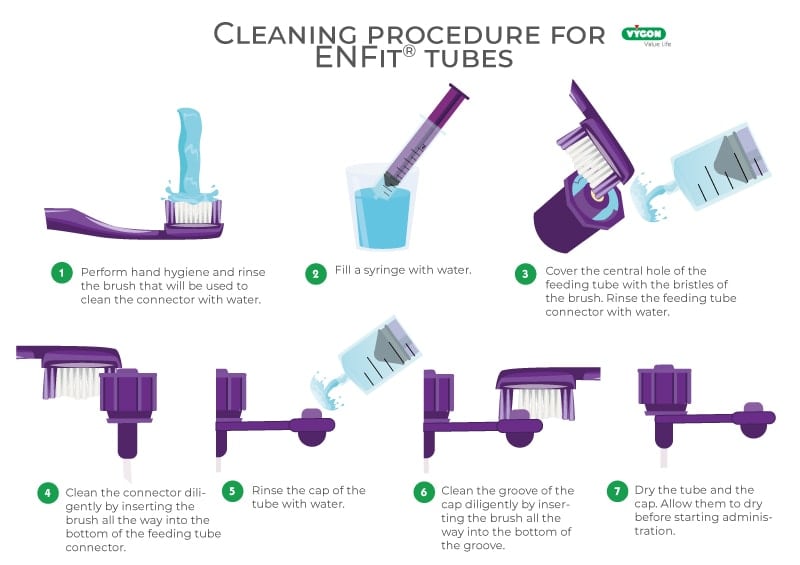
GEDSA cleaning protocol
Due to fluid displacement when making connections with ENFit®, residue of the solution inevitably remains adhered to the edge of the connector or the threaded cap that protects it from external pathogens (1).
In view of this problem, GEDSA, the World Association of Enteral Device Suppliers, has been obliged to publish a cleaning procedure protocol which is mandatory before starting nutrition with the ENFit® connector, and which must be repeated at least every 24 hours or whenever it is visibly dirty (1,2).
Are hygiene protocols sufficient?
In response to these new cleaning procedures, studies have been conducted to reveal what exactly happens when ENFit® connectors are used. In one of the investigations analyzed, a set of 120 nurses divided into two groups applied different cleaning procedures for the ENFit® connector. One group uses the protocol mentioned above; the other uses a more extensive procedure with 17 steps.
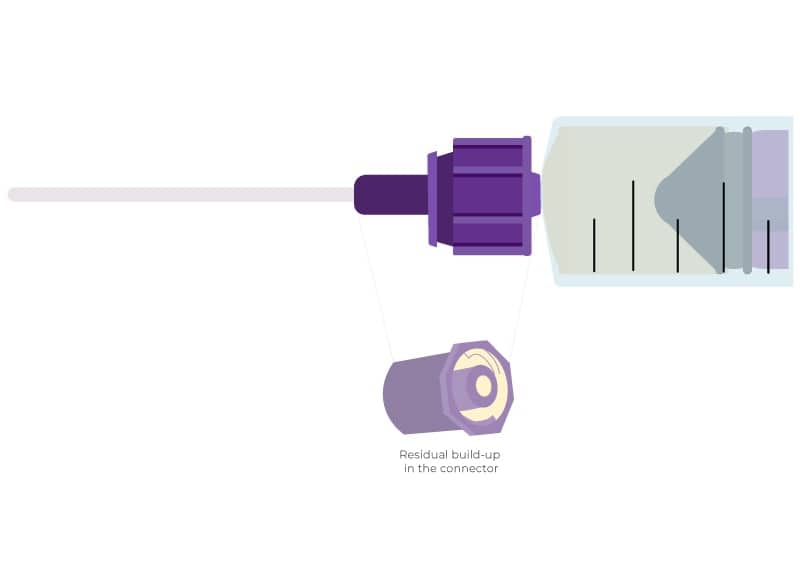
The test results showed that an average of 70% of the connections showed traces of the formula even when the most diligent cleaning procedure had been applied. In the case of the less extensive cleaning protocol, the rate reached 87% of the connectors (1.3).
It should be noted that this investigation was a simulation under perfect conditions outside the NICU in which the professionals were only engaged in cleaning the devices. Therefore, it is understood that the percentages increase under normal conditions, i.e., with the usual care activity that exists in this type of unit.
With all this, we can assume that (1,2):
- The difficulty of cleaning connectors, derived from the data of the aforementioned study;
- The stress of care reduces the diligence of the cleaning processes, especially if they are extensive and complex procedures and the remains of formula are not clearly visible;
- As well as, the lack of habit or ignorance of the need and obligatory nature of applying hygiene protocols before starting administration, every 24 hours, – cleaning protocols for connectors intended for enteral nutrition were not applied prior to the appearance of ENFit®-, are three factors that increase the risk of moat’s connector contamination due to solution displacement.
Difficulty of cleaning associated with the risk of infection
The difficulty of cleaning the connectors of the ENFit® design is closely related to the increased risk of bacterial contamination of the device.
It must be taken into account that gastric tubes remain in place for several days. Unlike other enteral nutrition devices, the tubes are not changed daily in order to avoid the pain and stress of manipulations to the newborn. For this reason, keeping the connections in proper hygienic condition is a very important part of neonatal enteral feeding care. If formula accumulates in the tube connector without the possibility of completely removing the debris, as the mandatory cleaning protocols for this type of connections have proven to be totally ineffective, over time, breast milk/formula colonizes (1).
A study published by the journal Pediatric Research (2016) analysing 94 nasogastric nutrition tubes used in neonates concluded that nasogastric tubes produced high densities of bacteria even on the first day of use (< 24 hours). Eighty-nine percent of the probes produced more than 1000 colony forming units (CFU)/ml of bacteria, and 55% produced potentially pathogenic Enterobacteriaceae and/or Staphylococcus aureus (4).
Thus, when a tube is contaminated, a bolus of bacteria can be given at each meal, i.e., between 6 and 8 contaminated boluses can be administered to the patient. Bacteria in contaminated nasogastric tubes find an easy way into the body of the newborn, whose defenses against these types of pathogens is not developed, making them a patient prone to infection (1,4).
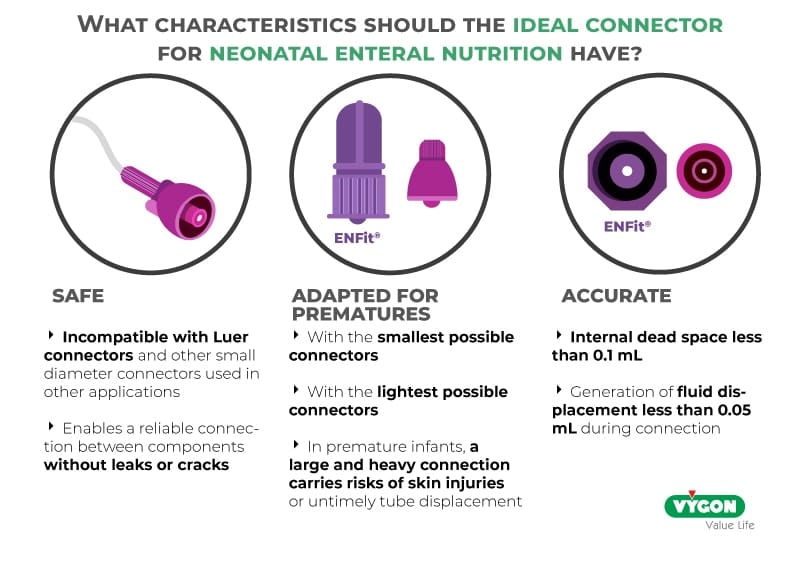
Safe connectors
To avoid possible consequences, such as infections, in vulnerable patients, it is recommended to choose neonatal enteral nutrition connections whose design does not require extra cleaning protocols, reduces the time invested by the nursing team in the optimal preservation of the connections and keeps the risk of contamination of the probes as low as possible, protecting the most fragile patients in the hospital (1).
Bibliography
- VYGON. (s. f.). Inquietudes. Safe Enteral. https://safe-enteral.com/es/low-dose-concerns/
- Resources. (2016, 21 marzo). StayConnected by GEDSA. https://stayconnected.org/resources/?language_category=es
- Lyman, B.et al. (2020). Randomized Controlled Trial Assessing the Effectiveness of Two Cleaning Regimens for ENFit®® Connectors. MedSurg Nursing, 29 (6).
- Petersen, S. M., Greisen, G., & Krogfelt, K. A. (2016). Nasogastric feeding tubes from a neonatal department yield high concentrations of potentially pathogenic bacteria— even 1 d after insertion. Pediatric Research, 80(3), 395–400. https://doi.org/10.1038/pr.2016.86 Ajouter au projet Citavi par DOI
Authors
Sales representative for Vygon Spain in the Basque Country, Cantabria, Rioja, Navarra, Burgos and Soria.
EXPERIENCE
I have a degree in Communication Sciences, branch Journalism, but I have developed my entire professional career in the commercial sector, health and biotechnology.
I CAN HELP YOU IN…
I can offer you help in choosing the medical-surgical material you need for your health care work.
Sales Representative – Cordoba, Malaga and Melilla in Vygon Spain
EXPERIENCE
I have been in hospital sales for 32 years, where I have held various positions, area manager for Andalusia, Extremadura and the Canary Islands, training and recruitment manager. I have been a Vygon sales representative for 10 years.
I CAN HELP YOU…
My work is based on advice on our products and procedural techniques. Please do not hesitate to contact me.