Enteral nutrition tubes are used in healthcare facilities to administer nutrition, fluids and medication to patients who cannot be fed orally.
For years, these patients have been subjected to the risks of misconnections, defined as incorrect administration of an enteral formula or drug.
In July 2016, the International Organisation for Standardisation (ISO) published the technical standard ISO 80369-3 defining a standardised safety connection for enteral nutrition. This new connection, called ENFit™, by means of a specific design, prevents the risk of accidental connection between different access routes (e.g. intravenous milk infusion).
While the ENFit™ connection solved the primary problem of patient safety, another problem emerged:
How to clean the distal end of the feeding tube that contains the large moat (figure 1), where residues of the administered solutions are deposited?


Figure 1. Residual nutrient solutions in the ENFit™ connection.
Under such conditions, the formation of biofilm, potentially responsible for the onset of infections, is encouraged.
Although this does not represent a major risk in the treatment of paediatric and adult patients, what is the impact in a more vulnerable population such as premature infants?
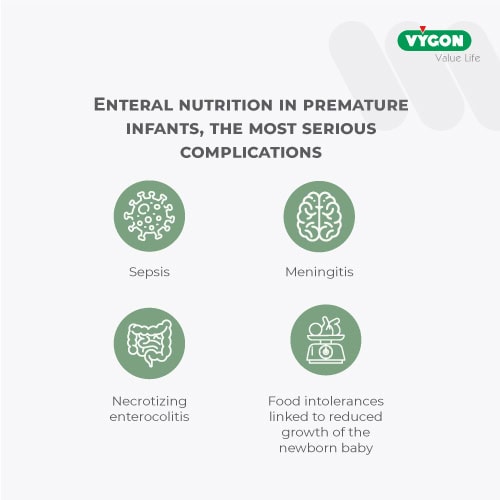
Enteral nutrition in premature infants
Infants in neonatal intensive care units often require nutrition through a feeding tube.
These tubes are in almost constant contact with the nutrient formula or breast milk, both of which are rich in sugars that promote bacterial development and growth.
It is estimated that biofilm can form on the surfaces of feeding tubes within the first 24 hours and, according to the literature, the dwell time of the tube does not correlate with the bacterial density found there.
Another aspect that may favour biofilm formation is the continuous handling of the tubes by healthcare workers, which occurs on average every 2-3 hours.
Bacterial contamination of enteral feeding tubes has been a topic discussed for years in the literature and has slowly been forgotten.
This is justified by the fact that in paediatric and adult patients the consequences of such contamination are clinically irrelevant.
However, the population of preterm infants is more vulnerable to the problem as, due to their still immature immune system and the absence of the intestinal microbiota, significant complications can occur.
Enteral connection cleaning protocols
There are currently no standard protocols for cleaning enteral connections.
The only recommendation in the literature suggests at least one cleaning per day.
Although there is no universally recognised approach to cleaning enteral connections, the rules of good practice call for the removal of visible residues until the device is deemed safe for use.
One must also consider the impossibility of using detergents and chemicals, which could be accidentally administered to the patient.
This makes the manoeuvre of cleaning enteral nutrition connectors even more complicated.
Improving cleaning protocols: a real solution?
In light of the above, it is evident that, in the neonatal population, clean enteral connections are not a negligible problem.
Can the implementation of more meticulous cleaning protocols therefore be a solution?
In a study published in 20205, this topic is addressed.
In particular, two cleaning protocols were analysed: a ‘more diligent’ one consisting of 17 steps and a ‘less diligent’ one consisting of 8 steps.
Two solutions were used to evaluate the effectiveness of the cleaning protocols, one visible to the naked eye and one visible only with UV light.
Below are the two protocols under study.
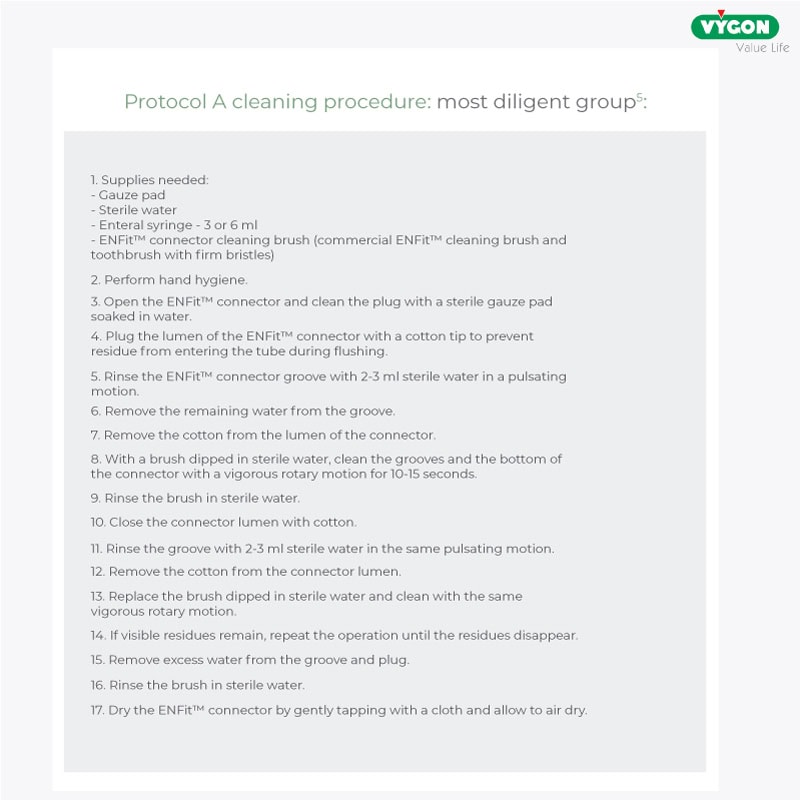

The results reported that even in the ‘more diligent’ protocol, although it ensured greater cleanliness than the ‘less diligent’ one, residues were found in about 70 per cent of the connections.
It has also been shown that the visualisation of the solution in the connector ensures better cleaning of the connector; therefore, it is recommended to carry out washing even in the absence of visible residues.
Although the results of the study did not identify any protocol that could eliminate residues in the connectors, a more elaborate protocol could increase cleanliness levels, reducing the occurrence of infections and complications in the neonatal population.
On the other hand, it must be considered that the increased complexity of the procedures has a strong impact on the workload of nurses, especially in neonatal wards where enteral tube manipulations occur frequently.
Once again, therefore, the ENFit™ connection seems not to be the best option for the neonatal population, and even more so for preterm infants.
For this patient population, there are safety connections with a design that offers more advantages than the ENFit™ design, such as the smaller connector size, the absence of threads and the more prominent external male lumen.
These characteristics ensure the treatment of newborns under safer conditions, on the one hand in terms of the accuracy of the dose administered and on the other hand in terms of reducing the risks of bacterial contamination.
Bibliography
- Mehall, J. R., Kite, C. A., Saltzman, D. A., Wallett, T., Jackson, R. J., & Smith, S. D. (2002). Prospective study of the incidence and complications of bacterial contamination of enteral feeding in neonates. Journal of Pediatric Surgery, 37(8), 1177-1182.
- Mehall, J. R., Kite, C. A., Gilliam, C. H., Jackson, R. J., & Smith, S. D. (2002). Enteral feeding tubes are a reservoir for nosocomial antibiotic-resistant pathogens. Journal of Pediatric Surgery, 37(7), 1011-1012.
- Parker, L. A., Magalhães, M., Desorcy-Scherer, K., Torrez Lamberti, M., Lorca, G. L., & Neu, J. (2022). Neonatal feeding tube colonisation and the potential effect on infant health: a review. Frontiers in Nutrition, 9, 775014.
- Guenter, P., & Lyman, B. (2016). ENFit™ enteral nutrition connectors: benefits and challenges. Nutrition in Clinical Practice, 31(6), 769-772.
- Lyman, B. (2020). Randomized Controlled Trial Assessing the Effectiveness of Two Cleaning Regimens for ENFit™® Connectors. MedSurg Nursing, 29(6).
- U.S. Food and Drug Administration (FDA). (2017). Reprocessing medical devices in health care settings: Validation methods and labeling. Guidance for industry and Food and Drug Administration staff. https://www.fda.gov/media/80265/download