For the treatment of patients, from adults to infants and premature babies, admitted to health care facilities, it is often necessary to place probes and catheters, the use of which involves the administration of enteral nutrition in addition to the infusion of drugs and other therapies.
The connections of these different delivery systems are often compatible with each other and may be unintentionally interconnected. Phenomena of this kind are identified as accidental connections of infusion lines. If this kind of error occurs in the management of infants, the consequences may be even more serious than in adults. Furthermore, it is important that medical devices used to administer enteral nutrition in neonates take into account their specific needs and characteristics, which are hardly comparable with those of adult patients.
The problem of accidental connections in enteral nutrition of the newborn
The definition of accidental connection in healthcare refers to seemingly incompatible medical devices that, if inadvertently connected, may cause adverse and dangerous events or even death to the patient.
The literature reports numerous examples of accidental connections, including connections between enteral feeding probes and intravenous (IV) lines, blood pressure sleeves and IV lines or IV lines and tracheostomy probes.
In this article, we will deal in particular with accidental connections in the context of enteral nutrition, i.e. the clinical procedure involving the administration of nutrients through specific probes inserted into the digestive system, focusing on newborns and what is the best solution to reduce the risk of accidents in such fragile and at-risk patients.
Cases of accidental connection between these lines and an intravenous catheter are extensively documented in the literature due to their particularly dangerous nature, ranging from therapeutic delay in the case of enteral administration of IV drugs to the death of the patient in the case of administration of IV nutrient mixtures.
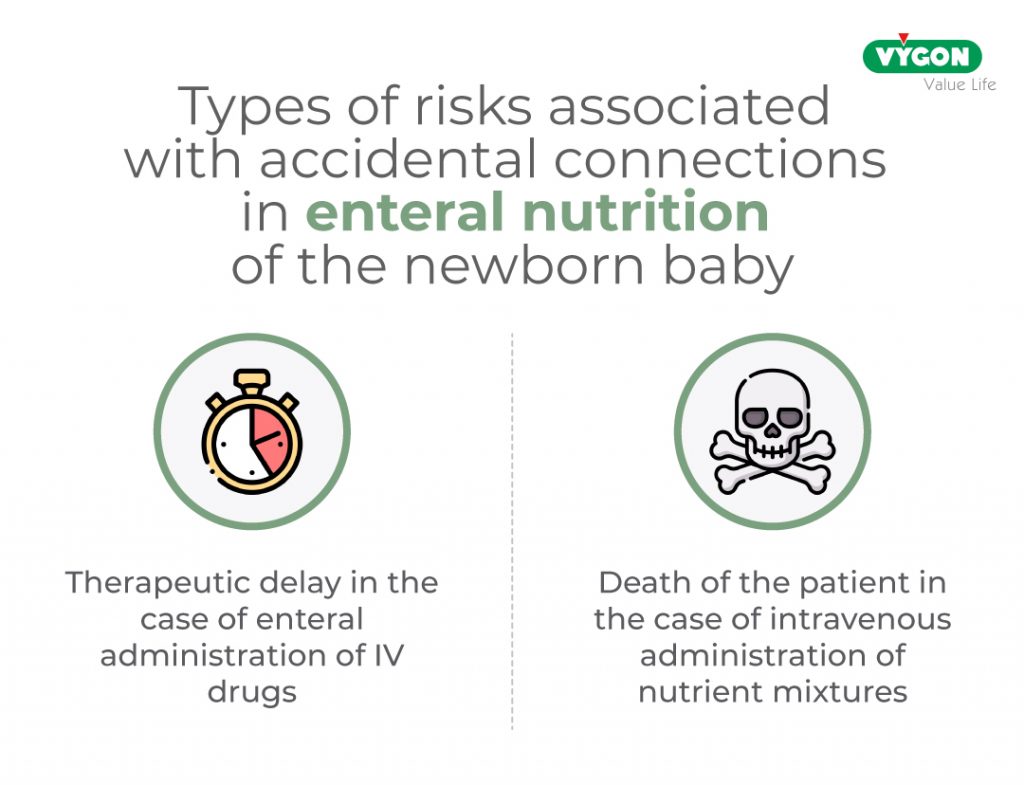
The adverse effects of this accidental connection increase exponentially when the subject is newborn or premature due to the particular fragile condition of this patient.
What are the factors contributing to the occurrence of a connection error?
These events are caused by the adverse conditions found in neonatal intensive care units, where a number of factors favour the occurrence of this type of incident:
- Babies are subjected to constant manipulation that increases the risk of human error;
- development and research in the neonatal field have made it possible for newborns to survive even in very unfavourable conditions, which require a large number of medical device connections for their treatment, as well as the existence of various accesses for different routes of administration, which can also be interconnected;
- In neonatal intensive care areas, clinicians are subjected to high pressure and stress situations due to the vulnerability of the patients treated and the occurrence of sudden emergencies;
- possible presence of professionals who have not yet received the necessary training to care for a critical patient such as a premature infant;
- the environmental conditions of the Neonatal Intensive Care Unit that can occur (e.g. lack of light) mean that some errors go unnoticed;
- the patient’s inability to communicate increasing the risk of errors.
Human errors and system malfunctions are inevitable and make it necessary to create prevention mechanisms, such as the adoption of protocols or safety devices, in order to be able to handle them in the best possible way, should they occur, but above all to create a culture of error prevention, since, unfortunately, there is not always a solution to these errors, which can be fatal for the patient.
These consequences directly affect patients’ morbidity and mortality, causing embolisms, sepsis, acute cardiac and respiratory failure, permanent neurological damage and even death.
Accidental connections, is this an actual problem?
A literature review reports more than 60 citations concerning accidental enteral connections, confirming the seriousness of this type of error that often leads to the death of patients due to embolism or sepsis. The first reports date back to the early 1970s until a dramatic incident in Italy in 2012. In this case, milk was mistakenly infused into the vein of an infant, causing his death.
As early as 1986, several public and private organisations issued safety warnings about the potential and actual risk of connection errors in medical devices. However, the number of cases continued to increase.
Ten years later, in response to warnings and the publication of new cases, the AAMI (Association for the Advancement of Medical Instrumentation®) convened a group of experts to draw up safety requirements for connectors and adaptors in enteral nutrition equipment. The resulting standard – reaffirmed in 2005 – recommended that adapters and connectors used in the enteral nutrition system should be incompatible with female Luer-lock connectors. However, no alternative design standards based on this text were developed at that time.
A report on medical errors, published by the United States Pharmacopeia (USP) in 2017, reported 24 incidents of incorrect administration of enteral nutrition formulas from 2000-2006. Although the volume of cases was not excessively high, the level of severity associated with the error was critical in that it had resulted in permanent injury or death.
Faced with this dramatic situation, the health world has realised the importance of implementing a series of measures to put an end to these unfortunate connection errors.
The ISO standard for enteral nutrition
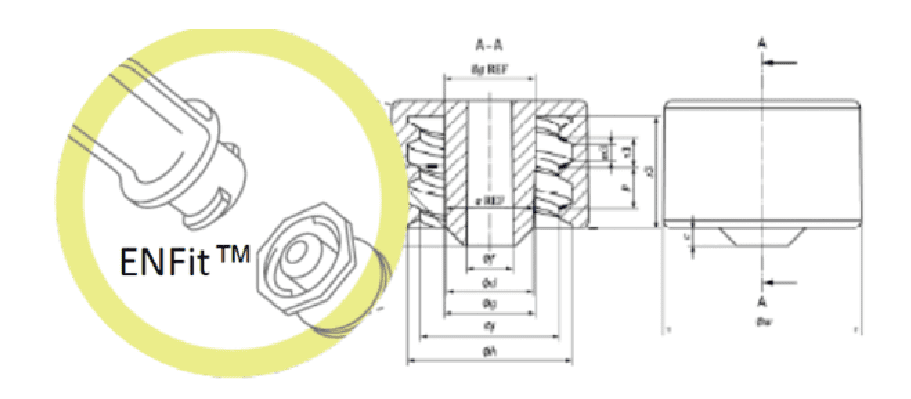
In July 2016, the International Organisation for Standardisation (ISO) published the technical standard ISO 80369-3 defining a standardised safety connection for enteral nutrition. This new connection, called ENFit™, by means of a specific design, prevents the risk of accidental connection between different access routes (e.g. intravenous milk infusion).
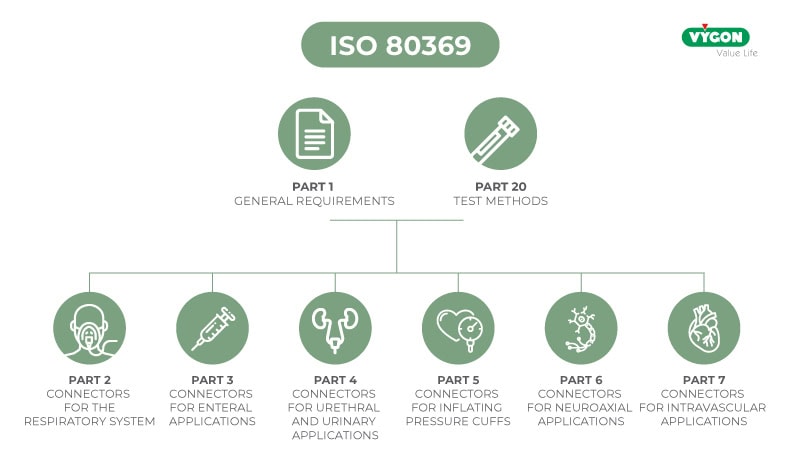
Part 3 of ISO 80369 specifies both how the connector shape must be structured and the dimensions and performance that the new enteral connector design must meet. This improves patient safety and allows standardisation of the design: incompatible with Luer or other small diameter connectors, increasing patient safety by avoiding possible connection errors (and preventing possible accidents and their consequences).
Once the general problem of connection error between infusion lines has been solved, knowing that neonates admitted to the neonatal intensive care unit have very specific needs, a question arises: is the design of the ENFitTM connection suitable for this patient population?
Concerns in the use of ENFIT™ in newborns
The ENFit connector has no technical or functional differences according to patient size, i.e. it is the same connector for both adult and neonatal or premature patients. The French Society of Neonatology, as soon as the specifications of ENFit were made known, alerted the ISO commission to the potential risks for the neonatal population of a connector that did not take into account its specific requirements in terms of size and infusion accuracy.
Want to learn more about the problem of using the ENFit connection in the newborn and how it was solved? Read the second part of the article: ‘Enteral nutrition in newborns: small connections for small patients‘.
Bibliography
- Lama, R. Nutrición Enteral. Revisado 20 Octubre 2019, en https://www.aeped.es/sites/default/files/documentos/5-nutricion_enteral.pdf Klaassen, J., García, P., Maíz, A., & Campano, M. (2002).
- Mecanismos de contaminación de las fómulas para nutrición enteral. Scielo: Revista Chilena De Infectología, 19 (. Revisado en: https://scielo.conicyt.cl/scielo.php?script=sci_arttext&pid=S0716-10182002000200001
- Lalueza, M., Rodríguez, V., Robles, A., & Fontán, C. (2019). Contaminación de nutriciones enterales en paceintes críticos. Validación del proceso de manipulación. Elsevier, (23). Revisado en: https://www.elsevier.es/es-revista-farmacia-hospitalaria-121-articulo-contaminacion-de-nutriciones-enterales-en-13005175
- Zúñiga, L., Rodríguez, M., & Hernández, T. (2017). Cuidados al paciente con nutrición enteral (NE). Gerencia de Atención Especializada de Medina del Campo. Revisado en: https://www.saludcastillayleon.es/investigacion/es/banco-evidencias-cuidados/ano-2017.ficheros/1204875-2017%20Protocolo_%20Nutricion%20enteral-%20envidencia.pdf
- Stevenson, J. (2008). Enteral Feeding Misconnections: A Consortium Position Statement [PDF] (5th ed., pp. 285-292). Revisado en: https://rdcms-aami.s3.amazonaws.com/files/production/public/FileDownloads/HT_Smallbore/TJC_S5-JQPS-05-08-guenter.pdf
- Malek, H., & Navarro, P. (2018). Nutrición Enteral: De la preocupación a la norma ISO 80369-3, ENFit – Campus Vygon. Revisado 22 Septiembre 2020, en https://campusvygon.com/global/iso-803693-enfit-nutricion-enteral/
- Extract ISO 80369-3 – Annexe E – Page 25. (2020). [Ebook]. Revisado en: https://www.iso.org/obp/ui/#iso:std:iso:80369:-3:ed-1:v1:en
- Extract ISO 80369-3 – Annexe A – Page 7. (2020). [Ebook]. Revisado en: https://www.iso.org/obp/ui/#iso:std:iso:80369:-3:ed-1:v1:en
- Malek, H. (2018). ENFit un sistema incompatible con la seguridad neonatal. Revisado el 22 Septiembre 2020, en https://campusvygon.com/global/enfit-no-seguro-neonatos/
- Análisis “the report GEDSA Low Dose Syringe Accuracy test, 31 de Enero 2016, by SMTL” – SELECT Report – Revisado el :22 de Septiembre de 2020
- VYGON (2014). “Estudio neonatal sobre el impacto potencial del conector ENFit en la precisión de la jeringa” en Vygon.es. http://www.safe-enteral.com/es/neonatology-needs/ [Revisado: 7/08/2018].
- ISO 20695:2020 Enteral feeding systems – Design and testing. (2020). Retrieved 22 September 2020, from https://www.une.org/encuentra-tu-norma/busca-tu-norma/iso?c=068853
- Guía de GEDSA en respaldo a la norma ISO 80369-3 para ENFit®. (2017). [pdf].
- Chassin, M; Pujols-McKee, A. (2017). Reservas acerca del diseño del conector ENFit y de los riesgos de seguridad en las unidades de cuidados intensivos neonatales (UCIN). [NCfIH paper].
- http://www.safe-enteral.com/
- March 2016 newsletter of the French Society of Neonatology (SFN)
Authors
First Product Specialist, now Technical Information Specialist at Vygon Italia, I look after the technical documentation of our products. I studied in Rome at the University of Torvergata, where I graduated in Medical Engineering with an experimental thesis on aortic valve replacement. For some time now, I have been passionate about the world of Digital, a powerful tool that allows us to educate and inform ourselves, anytime and anywhere. My goal is to help disseminate the most up-to-date clinical practice, with quality content, so as to contribute, albeit indirectly, to patient well-being.
I am the Business Unit Manager of the neonatology, enteral nutrition and obstetrics lines for Vygon Italy. I am an electronics-biomedical engineer and have been cultivating a strong interest in marketing, technology and innovation in the medical device field for more than 18 years. Today, I am proud to be able to put my professional experience and Vygon’s know-how at the disposal of healthcare professionals caring for newborns.