For the treatment of patients, from adults to infants and premature babies, admitted to healthcare facilities, it is often necessary to place probes and catheters whose use includes the administration of enteral nutrition in addition to the infusion of drugs and other therapies. The connections of these different administration systems are often compatible with each other and may be interconnected involuntarily.
In an attempt to solve this serious problem, in July 2016, the International Organisation for Standardisation (ISO) published the technical standard ISO 80369-3, which defines a standardised safety connection for enteral nutrition. This new connection, called ENFit™, through a specific design, prevents the risk of accidental connection between different access routes (example: intravenous milk infusion).
Infants and premature babies, however, are very special patients, fragile and with specific needs; therefore, a question arises: is the design of the ENFit™ connection suitable for this patient population?
Let’s see it together in this article!
Concerns in the use of ENFIT™ in newborns
The ENFit™ connector has no technical or functional differences according to patient size, i.e. it is the same connector for both adult and neonatal or premature patients. The French Society of Neonatology, as soon as the specifications of ENFit™ were known, alerted the ISO commission to the potential risks for the neonatal population of a connector that did not take into account its specific requirements in terms of size and infusion accuracy.
The concerns of the French Society of Neonatology, also reiterated in a newsletter of March 2016, are based on the evidence that the ENFit™ connector has a footprint (external) and dead space (internal) that is unsuitable for the neonatal environment, both for reasons of patient comfort and, above all, for the need for infusions that may require precision in the order of fractions of a millilitre (e.g. for digoxin, anticonvulsants, caffeine). During the discussion of the standard, several national harmonisation bodies, such as AFNOR (France), AENOR (Spain), BSI (UK) and UNI (Italy), acknowledged these concerns and requested further studies from the ISO commission.
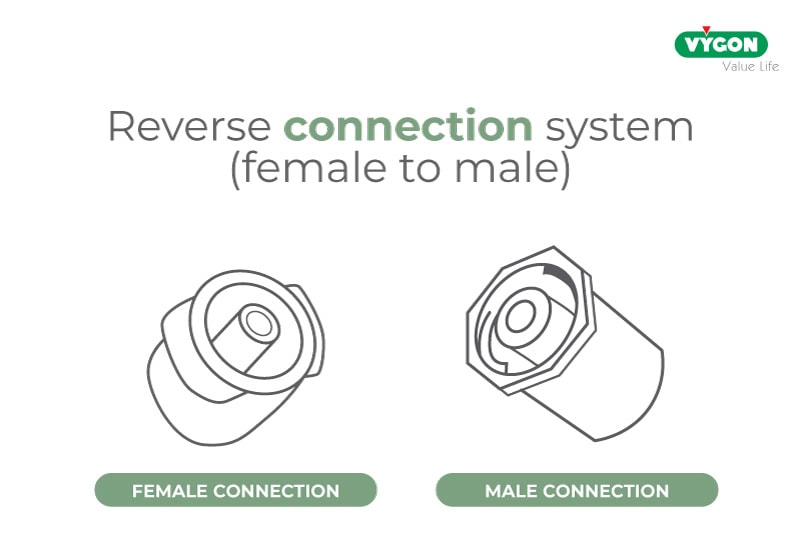
The ensuing investigations proved these concerns to be well-founded and led the ISO commission to highlight the potential risks to the neonatal population by including specific warnings in Annex A of ISO 80369-3, an excerpt from which is given below:
“Concerns have been expressed about the possible risks associated with inaccurate drug delivery in certain clinical practices on high-risk subpopulations, such as neonatal patients, when using an inverted (female to male) connection system. Such an orientation may cause accidental displacement of fluid originally contained in the […] syringe tip.”
Extract from ISO 80369-3 – Annex A – Subpopulations in enteral clinical application
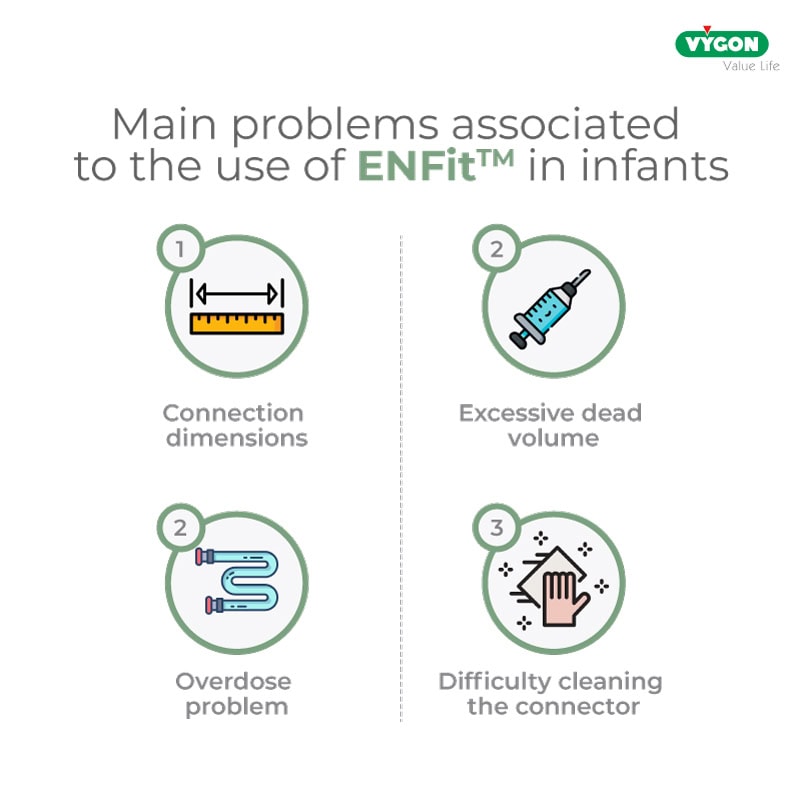
1. Dimensions of the ENFit™ connection
The excessive non-rounded size and weight of the standard ENFit™ connectors, when used in neonates admitted to the neonatal intensive care unit, pose the risk of skin lesions or untimely displacement of the enteral tube.

2. Excessive dead volume
Excess fluid accumulated in the ENFit™ connector can be problematic in a neonatal patient, where the dosages of solutions, and especially of drugs administered, are very low.

Indeed, if the cone is not cleaned properly, the infant may receive up to 30% more drug, with the risk of adverse reactions to the administered drug.
3. Overdose problem
Administering the correct dose to an infant is vital: too low a dosage may be ineffective, while too high a dosage may cause side effects that are also very harmful to the infant’s health, especially when using a high-risk drug.
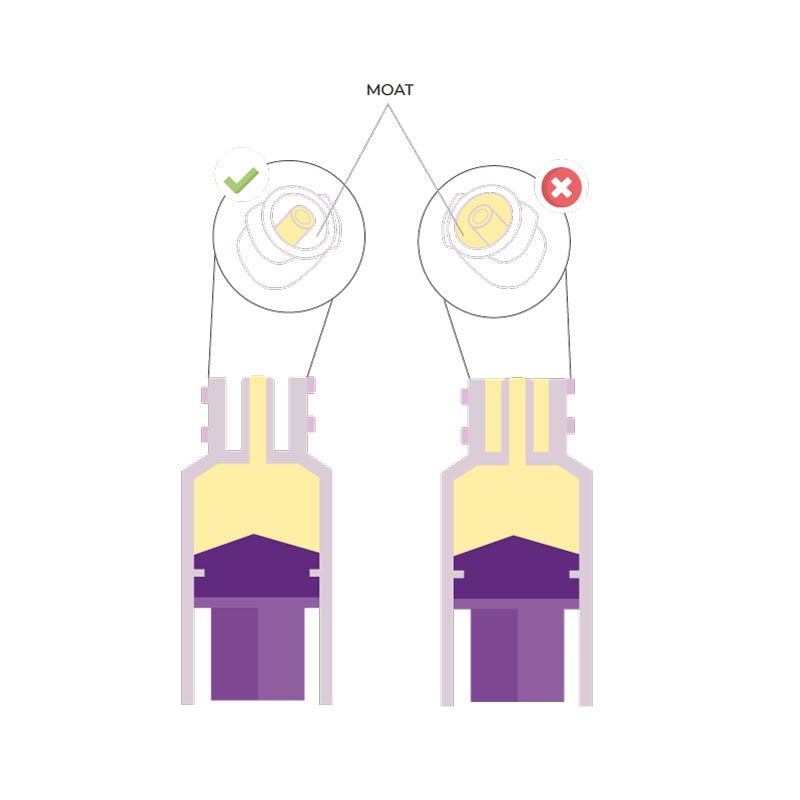
In this regard we quote an excerpt from ISO 80369-3:2016: ‘In a 500 g premature infant, enteral medications are often prescribed in very low dosages, in the range of 0.1 ml or even 0.01 ml’. However, ‘Laboratory tests show that a pair of medium tolerance [ENFit™] connectors coupled in a female-to-male orientation displaces an average fluid volume of 0.148 ml […]’.
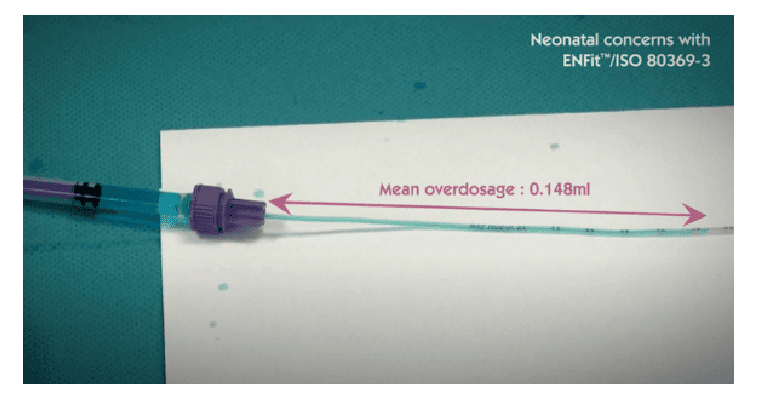
The Children’s Hospital of Philadelphia published data showing that more than 80 different oral therapies are administered in quantities of less than 2 ml and 1,250 of these doses are prepared daily, representing 48% of the daily oral doses administered.
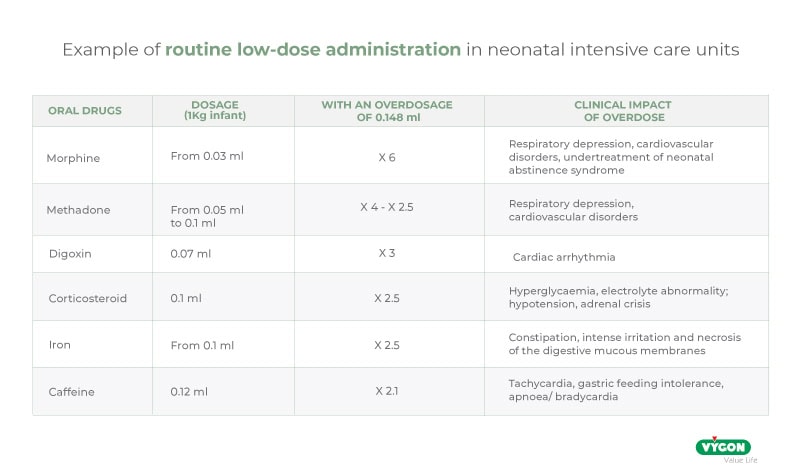
It is clear that an overdose of almost 0.15 ml can put the infant at serious risk and, therefore, the use of ENFit™ connectors in the neonatal population is not recommended.
LDT ENFit™ syringes: a real solution to the problem?
In an attempt to solve the problem, ENFit™ LDT (Low Dose Tip) type syringes were introduced onto the market. For such connectors, however, it was soon shown that the risk of overdose did not decrease.
Clarity is provided by the FDA, which issued a Safety Communication on the risk of overdose on 12 October 2021. This safety communication warns patients and healthcare professionals about potential overdoses when using ENFit™ low-dose syringes (LDT) due to their connector design.
“The Food and Drug Administration (FDA) is informing patients and healthcare providers about the potential risk of overdose, under certain clinical use conditions, when using ENFit™ low-dose tip (LDT) syringes.”
Laboratory tests have shown that the cavity of the low-volume syringe (LDT) can be unintentionally filled with fluid during withdrawal. Results show that the low-volume syringe (LDT) can lead to an overdose of 0.120 ml or even 0.153 ml in some cases, when fluid is present in the syringe cavity.
If we compare the overdose risk of each safety enteral feeding system using a numerical simulation, the results show that the low-dose syringe (LDT) can lead to an overdose equivalent to the conventional ENFit™ syringe.
Today, even the IFUs of some ENFit™ syringes contain recommendations on how to use ENFit™ LDT syringes to avoid the risk of overdose, for example:
“- Use a straw or bottle adapter to fill the syringe to prevent excess residual fluid from escaping the fluid path. – Check that the channel is free of any excess fluid prior to administration. – Slightly tap/stroke the syringe to displace any fluid that may be outside the fluid path. – In the event of improper use, the risk of overdose will average 0.120 ml (as calculated by a numerical simulation).”
As a solution to this problem, GEDSA, the Global Association of Enteral Device Suppliers, recommends that manufacturers include in the management protocol of enteral nutrition devices, an essential step of cleaning the syringe prior to administration, thus resulting in a change to the protocol and an increase in the workload of the healthcare personnel involved.
4. Difficulty in cleaning the connector
The large size of these connections complicates the cleaning procedure of the devices. Indeed, some are designed with a margin outside the line where liquid can accumulate, making it difficult to clean syringe cones and maintain feeding tubes, increasing the risk of infection. Indeed, if the residual infusion solution in the connector is not removed, it runs the risk of bacterial contamination. Therefore, the risk of infection in the neonate due to device contamination increases with the difficulty of cleaning the syringe connector and/or ENFit™ tubes.
The difficulty in having to clean such devices does not only entail the risk of overdose or contamination of the enteral solution, but also entails having to change the usual processes of dedicated personnel. Professionals have to deal with additional processes for the regular cleaning of all enteral devices, in addition to the use of various connectors and adapters for oral or enteral drug administration.
These additional procedures for cleaning tubes and accessories require changes in the usual work protocols, hence additional and appropriate training for all departments/functions, with a significant additional burden in terms of time and costs.
The advantages of a dedicated enteral connection in the newborn
As suggested by the French Society of Neonatology (SFN) in its March 2016 newsletter, the solution to all these problems is to adopt a safe enteral system that is specific to newborns and premature infants.
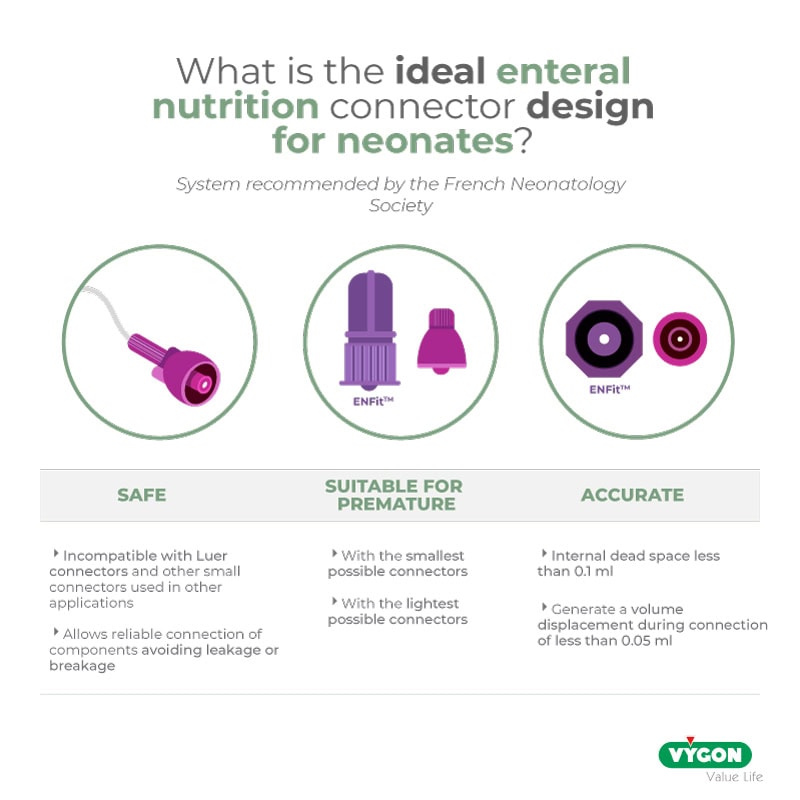
This system must have certain fundamental characteristics:
- must be SAFE, i.e. incompatible with Luer connectors and other small connectors used in other applications (e.g. intravascular) described in the ISO 80369 standard and designed to allow reliable connection of components without leakage or breakage (compatibility with 80369-1 standard);
- it must be PRECISE, i.e. it must ensure the highest dosing accuracy, which is essential for administering small doses of drugs, have an internal dead space of less than 0.1 ml and generate a volume displacement during connection of less than 0.05 ml (data from the SFN newsletter);
- must be SMALL and of a size suitable for the neonatal population, specifically designed and engineered for use in neonates and preterm infants, minimising the size, weight and dead space of the connection.
From the beginning, many professionals welcomed this transformation of the usual routine, because they were aware of the benefits it would bring and it meant a change towards a culture of safety. As with any change, there was a learning curve, but it was not overly complicated as the benefits of this were obvious, both in terms of workflow and patient safety.
ISO 80369-3 is not a mandatory standard, it is therefore up to each department to adopt or not to adopt the ENFit™ safety system, even more so to use a safety system that is also dedicated to neonates. If you still have doubts about the advantages of adopting an enteral system dedicated to newborns, below you will find the position on this issue of many associations and scientific societies.
Are the safety enteral feeding systems accurate enough for neonatal drug administration? By Dr O’Mara
Dr O’Mara gave a symposium at the joint European Neonatal Societies Virtual Congress in September 2021. She addressed an important topic in neonatology: is the safety enteral nutrition system accurate enough for neonatal drug administration? This is a key question for premature infants in the neonatal intensive care unit, Dr. O’Mara presented the results of her studies.
At the last ‘NICU leadership forum’ held in Florida, the risks associated with the use of ENFit™ in neonatology were discussed
In April 2018, during the 22nd ‘NICU Leadership Forum’ in Ponte Vedra Beach, Heidi McNeely (neonatal nurse, Colorado), Susan Hepworth (director of the National Coalition for Infant Health – NCfIH) and Keliana O’Mara (pharmacist, Florida) made a group presentation to inform about the risks of using the ENFit™ system in neonatal services.
The connector of the classic ENFit™ or ‘Low Dose Tip’ syringe is difficult to clean and increases the risk of infection and inaccurate dosing.
The workload of nurses and pharmacists has increased.
Poor dosage accuracy can lead to overdose and adverse drug reactions.
The national coalition for infant health (NCFIH) has issued a warning letter on the use of the ENFit™ system in neonatology
In late 2017, NCfIH wrote a letter to the Joint Commission outlining its concerns and the dangers of using the ENFit™ system on newborns.
In particular, the organisation emphasises that in neonatology it is extremely important to administer small quantities of drugs with maximum precision.
The French society of neonatology (SFN) recommends a specific safety enteral system for neonates
In its March 2016 newsletter, the SFN warns French neonatologists about the risk of inaccurate dosing of ENFit™ systems and recommends a neonatal enteral nutrition system that is:
SAFE
Incompatible with Luer connectors and other small connectors used in other applications (e.g. EV)
Designed to allow reliable connection of components avoiding leakage or breakage
PRECISE
With an internal dead space of less than 0.1 ml
Generate a volume displacement during connection of less than 0.05 ml
Of a size suitable for use with preterm infants
Incorporate the smallest possible connectors
Incorporate the lightest possible connectors
Joint commission responds to NCFIH and reassures healthcare professionals
In its response, the Joint Commission assures that it does not impose any obligations in terms of the use of medical devices or other technologies. Healthcare facilities are free to determine which products are best suited to their patients. This allows healthcare facilities to continue to protect their most vulnerable patients from the risks of inaccurate dosing by using devices of their choice. The NCfIH takes a very positive view of the response.
The Institute for Patient Access (IFPA) also denounces the dangers caused by the ENFit™ system
As Suzanne Staebler, a neonatal nurse in Atlanta with 25 years of work experience, explains, healthcare professionals face new challenges caused by the ENFit™ connector and the ‘Low Dose Tip’ syringe:
- For neonatal and paediatric patients, this design does not guarantee the dosing accuracy required for treatment.
- Staff must also take responsibility for cleaning the ENFIT™ ‘Low Dose Tip’ syringe connector to remove any excess drug, which is not always evident.
Other professionals are concerned
The Working Group of the Canadian Standards Agency (CSA Z298) identified serious shortcomings in ISO 80369-3 and voted against this draft standard. One of the technical reasons for this lack of support is the imprecise dosage of the ENFit™ system.
BIBLIOGRAPHY
- Lama, R. Nutrición Enteral. Revisado 20 Octubre 2019, en https://www.aeped.es/sites/default/files/documentos/5-nutricion_enteral.pdf Klaassen, J., García, P., Maíz, A., & Campano, M. (2002).
- Mecanismos de contaminación de las fómulas para nutrición enteral. Scielo: Revista Chilena De Infectología, 19 (. Revisado en: https://scielo.conicyt.cl/scielo.php?script=sci_arttext&pid=S0716-10182002000200001
- Lalueza, M., Rodríguez, V., Robles, A., & Fontán, C. (2019). Contaminación de nutriciones enterales en paceintes críticos. Validación del proceso de manipulación. Elsevier, (23). Revisado en: https://www.elsevier.es/es-revista-farmacia-hospitalaria-121-articulo-contaminacion-de-nutriciones-enterales-en-13005175
- Zúñiga, L., Rodríguez, M., & Hernández, T. (2017). Cuidados al paciente con nutrición enteral (NE). Gerencia de Atención Especializada de Medina del Campo. Revisado en: https://www.saludcastillayleon.es/investigacion/es/banco-evidencias-cuidados/ano-2017.ficheros/1204875-2017%20Protocolo_%20Nutricion%20enteral-%20envidencia.pdf
- Stevenson, J. (2008). Enteral Feeding Misconnections: A Consortium Position Statement [PDF] (5th ed., pp. 285-292). Revisado en: https://rdcms-aami.s3.amazonaws.com/files/production/public/FileDownloads/HT_Smallbore/TJC_S5-JQPS-05-08-guenter.pdf
- Malek, H., & Navarro, P. (2018). Nutrición Enteral: De la preocupación a la norma ISO 80369-3, ENFit – Campus Vygon. Revisado 22 Septiembre 2020, en https://campusvygon.com/global/iso-803693-enfit-nutricion-enteral/
- Extract ISO 80369-3 – Annexe E – Page 25. (2020). [Ebook]. Revisado en: https://www.iso.org/obp/ui/#iso:std:iso:80369:-3:ed-1:v1:en
- Extract ISO 80369-3 – Annexe A – Page 7. (2020). [Ebook]. Revisado en: https://www.iso.org/obp/ui/#iso:std:iso:80369:-3:ed-1:v1:en
- Malek, H. (2018). ENFit un sistema incompatible con la seguridad neonatal. Revisado el 22 Septiembre 2020, en https://campusvygon.com/global/enfit-no-seguro-neonatos/
- Análisis “the report GEDSA Low Dose Syringe Accuracy test, 31 de Enero 2016, by SMTL” – SELECT Report – Revisado el :22 de Septiembre de 2020
- VYGON (2014). “Estudio neonatal sobre el impacto potencial del conector ENFit en la precisión de la jeringa” en Vygon.es. http://www.safe-enteral.com/es/neonatology-needs/ [Revisado: 7/08/2018].
- ISO 20695:2020 Enteral feeding systems – Design and testing. (2020). Retrieved 22 September 2020, from https://www.une.org/encuentra-tu-norma/busca-tu-norma/iso?c=068853
- Guía de GEDSA en respaldo a la norma ISO 80369-3 para ENFit®. (2017). [pdf].
- Chassin, M; Pujols-McKee, A. (2017). Reservas acerca del diseño del conector ENFit y de los riesgos de seguridad en las unidades de cuidados intensivos neonatales (UCIN). [NCfIH paper].
- http://www.safe-enteral.com/
- March 2016 newsletter of the French Society of Neonatology (SFN)
Authors
I am the Business Unit Manager of the neonatology, enteral nutrition and obstetrics lines for Vygon Italy. I am an electronics-biomedical engineer and have been cultivating a strong interest in marketing, technology and innovation in the medical device field for more than 18 years. Today, I am proud to be able to put my professional experience and Vygon’s know-how at the disposal of healthcare professionals caring for newborns.
First Product Specialist, now Technical Information Specialist at Vygon Italia, I look after the technical documentation of our products. I studied in Rome at the University of Torvergata, where I graduated in Medical Engineering with an experimental thesis on aortic valve replacement. For some time now, I have been passionate about the world of Digital, a powerful tool that allows us to educate and inform ourselves, anytime and anywhere. My goal is to help disseminate the most up-to-date clinical practice, with quality content, so as to contribute, albeit indirectly, to patient well-being.





