The following article offers an in-depth analysis of the scientific evidence on enteral nutrition in infants, focusing in particular on the methods of administration.
Nutrition in infants is a vital component for their development and well-being, and the choice of the right device plays a key role in ensuring adequate nutritional intake during the first moments of life.
Regulatory landscape and the ENFITTM connection
Inadvertent administration of enteral/oral fluids via the intravenous route can have serious consequences.
To this end, ISO 80369 defined international design standards exclusively for connectors for each healthcare application (respiratory, EV, enteral, etc.), making them not compatible with each other.
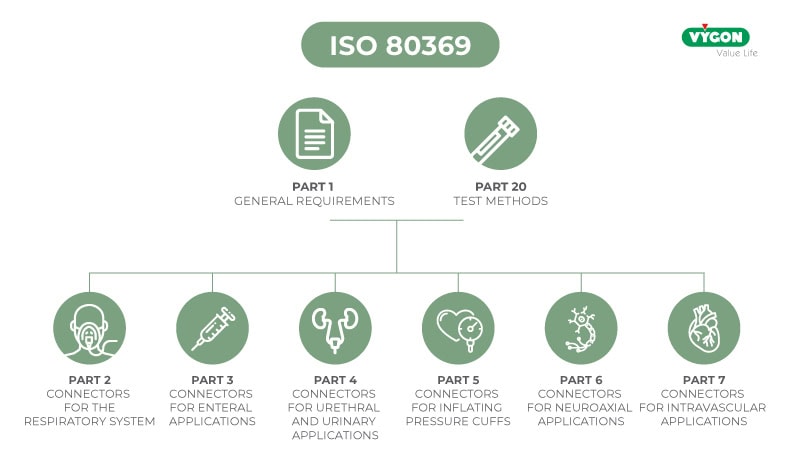
Part 3 of ISO 80369 defines the design and required dimensions of the ENFitTM connector dedicated to enteral application.
- Defines shape and size of ENFIT™ connector
- Defines functional performance of the ENFitTM connector
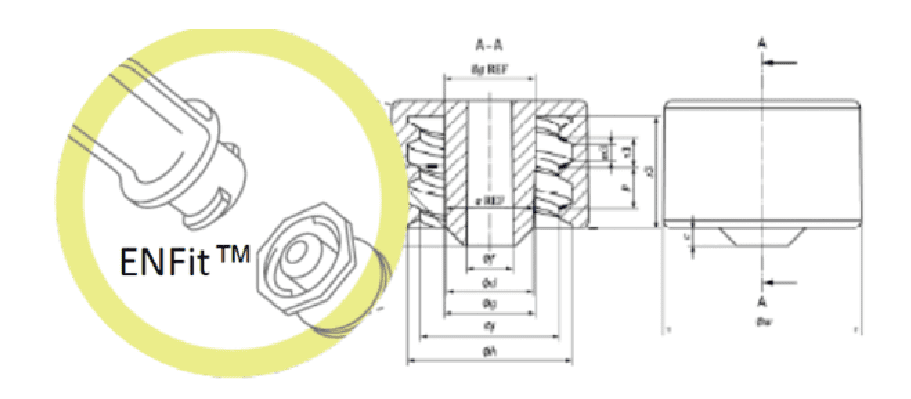
The female ENFitTM syringe has been proposed as a solution to connection errors on the enteral route. However, clinicians have seen that the tip structure of this type of syringe creates a physical space that can lead to inaccurate dosages of enteral solutions being administered (1,2).
The displacement volume of this type of syringe is 0.2 mL, which can lead to significant over- or underdosing, which is not particularly dangerous when it comes to an adult patient.
On the other hand, in the case of newborns and premature infants, the displacement volume when connecting is very important since, due to their low weight, even as low as 500 g, enteral medication is often prescribed in low quantities such as 0.1 ml or even 0.01 ml.
The Low Dose Tip syringe was designed to overcome the overdose problems of the ENFitTM but post-marketing clinical evaluations have shown that this syringe cannot guarantee the accuracy of a low dose (2-4).
The design and adoption of a safety connector for enteral nutrition is an outstanding and fundamental achievement to ensure the best possible care for all patients, but the use of a single safety connector, the same for all, from adult to premature infant, may not be the best choice.
In this regard, Dr. O’ Mara (from the Department of Pharmacy at WakeMed Health and Hospitals, Raleigh, NC) has often addressed this important topic in neonatology:
Is the ENFitTM safety enteral nutrition system accurate enough for the administration of neonatal drugs?
And this is a key question for all newborn and premature babies admitted to the neonatal intensive care unit, which demands a clear and detailed answer.
Scientific evidence
The most recently published article on the subject (June 2023), ‘Comparison of Dosing Accuracy Between the ENFittm LDT and a Neonatal-Specific ISO-Compliant Enteral Syringe‘ (8), deals with this sensitive topic, focusing on two different devices for the administration of enteral nutrition:
the ENFitTM safety connector with LDT (Low Dose Tip) and the NS2 infant and preterm safety connector.
This study aims to explore the various modes of administration of enteral nutrition, assessing their efficacy, safety and clinical implications. Through a critical analysis of the available scientific evidence, best practices in the field of neonatal enteral nutrition will be highlighted, with an emphasis on the clinical aspects for newborn health.
In this study in particular, the performance of the syringe with a dedicated connector for neonates and preterm infants, designed to the ISO 80369 safety standard, was evaluated against the ENFitTM LDT syringe.
Fluid dynamic studies suggest that the NS2 syringe can reduce the potential overdose seen with ENFitTM LDT from 0.13 ml to 0.029 ml due to the smaller syringe connector size.
Study materials and methods
This is an in vitro study performed at WakeMed Healt and Hospitals.
Two types of syringes, each of 3 different sizes, were evaluated. This choice was made on the basis of previous studies (5) which showed greater variability on syringes with small volumes:
- ENFitTM LDT 0.5 ml, 1 ml, 3 ml
- Neonatal safety connector NS2 0.5 ml, 1 ml, 2.5 ml
Two filling volumes were evaluated for each syringe: at 20% and 80% of total capacity.
To match the methodologies of the other studies and to represent a common outpatient drug, bromfeniramine maleate/dextromethorphan HBr/phenylephrine HCl oral (Dimetapp Cold & Cough for children; Pfizer Inc.) was used for all tests performed.
Results
300 tests were performed, 150 with the ENFitTM LDT syringe and 150 with the NS2 syringe. The FDA-recommended protocols were adhered to in all tests and the aim was to measure the accuracy of the administrations.
For each syringe size, half of the tests were carried out at 20% capacity and the other half at 80%.
The indicator analysed is the Dosing Variance (DV), defined as:
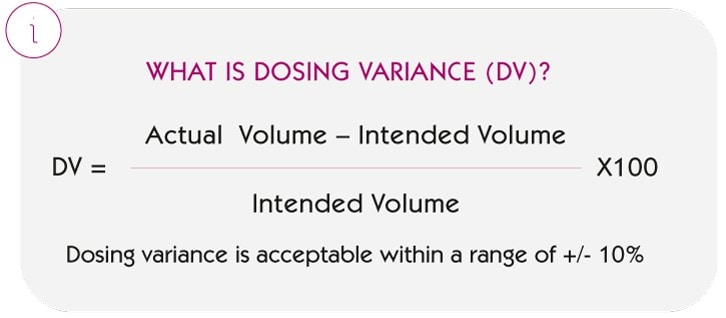
i.e. how much the volume actually administered deviates in percentage terms from what was intended to be administered.
In addition, both modes of administration were considered: with bottle adapters (bulk bottle adapters) and with graduated medication cups (medication cups).
The DV, compared to the total number of tests performed, was significantly higher in the LDT syringe group than in the NS2 group (11.9 % vs. 3.5 %)
Similar results were also obtained when considering different syringe measurements where, for all 3 measurements, the dedicated connector was more accurate than the ENFitTM LDT connector.
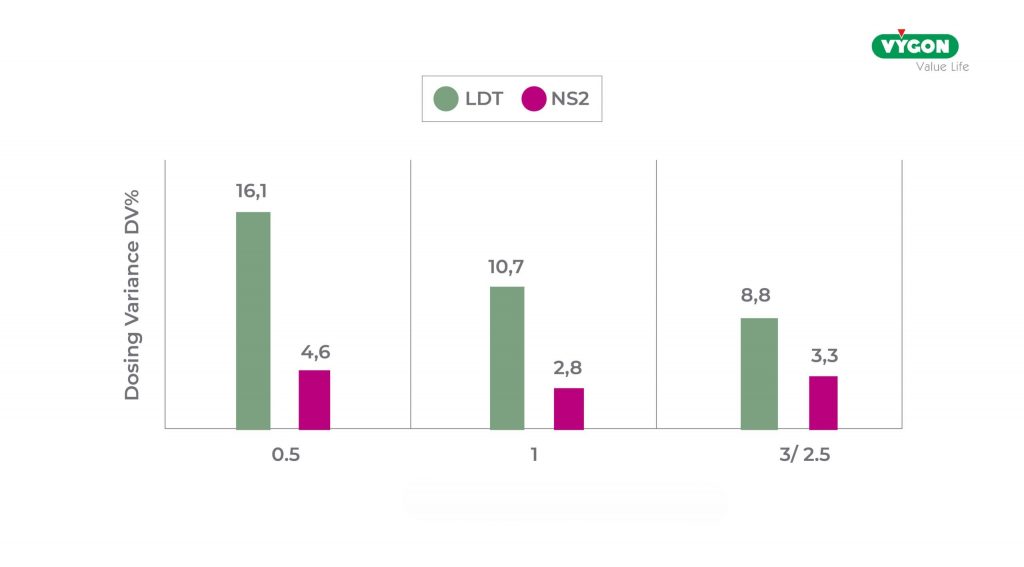
DV trend based on syringe size
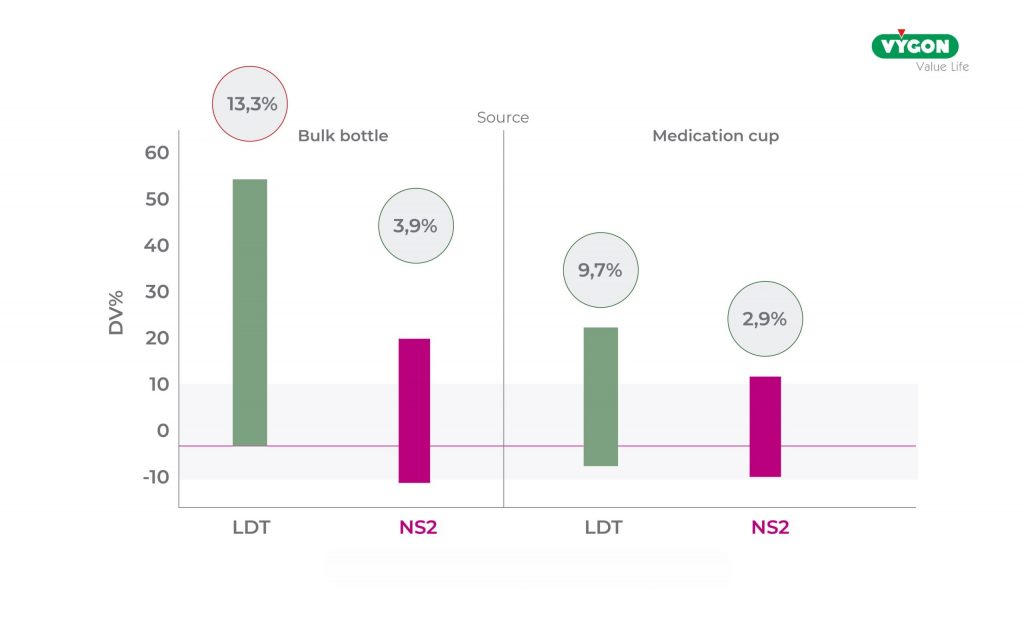
The accuracy of administration was also assessed according to the method by which the drugs were administered:
- Despite the use of bulk bottle adapters, the DV of the ENFitTM LDT syringes remains much higher than that of the NS2 syringes (13.3% vs. 3.9%) and well above acceptable values.
- whereas when using graduated medication cups an improvement in DV was found for both syringes (9.7% LDT Vs 2.9% NS2), which was acceptable but very close to the upper limit of 10% for ENFitTM LDT.
Type of syringe connector
Discussion
The choice of the most appropriate design for the enteral nutrition of neonatal and preterm patients must be made taking into account both the risk of incorrect dosage and the risk of incorrect connection.
Based on this, NS2 syringes show significantly higher dosing accuracy when compared to ENFitTM LDT.
A large portion of tests performed with ENFitTM LDT are associated with an even unacceptable DV of the administration volume, as well as higher than that associated with NS2; this result is consistent with previous evaluations (5-7).
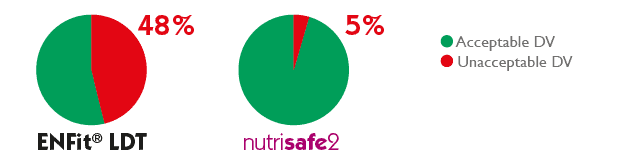
The overall results of the 150 tests performed for each syringe type showed that for ENFitTM LTD syringes, in 48% of cases, unacceptable results in delivery accuracy were encountered, compared to only 5% for syringes with dedicated neonatal connector.
Furthermore, a recent publication from 2022 (7) confirms the possibility that ENFitTM LDT may contribute to an unwanted overdose. In the study, an overdose of 34.7% was expected for 0.5 ml syringes, which is quite comparable to the experimental results showing an overdose of 39.6%. For the 1 ml syringe the estimated overdose was 18.1% which is within the experimental values (10.5%-26.4%). When creating a fluid displacement volume, fluid-dynamic simulations predicted an overdose of up to 300% of the predicted dose with the 0.5 ml ENFitTM LDT and 148% for the 1 ml.
Although this study adds to the literature on the dosing accuracy of enteral devices, it was not possible to evaluate certain parameters such as drug properties (viscosity and surface tension), pressures within the syringe and the fittings themselves, and the use of filling adapters, all of which influence flow behaviour. Indeed, computational studies have identified the geometry and fittings of the ENFitTM LDT syringe itself as the only source of inadequacy.
So for those who choose to use ENFitTM LDT syringes, it is crucial to mitigate the risk of overdose for safe drug administration.
Conclusions
- This study further validates that ENFitTM LDT syringes have a higher risk of dose inaccuracy than other available syringe models.
- The syringe with a dedicated connector for neonates and premature infants certainly shows an advantage in terms of dosing accuracy due to its specific design and small size, significantly lowering the maximum volume of drug that can be administered unintentionally.
- Smaller syringes are generally associated with increased dosage inaccuracy, which emphasises the need to develop oral drugs with appropriate concentrations and dosages for infants.
When a new enteral nutrition device enters the market, it is crucial to test its safety, precision and accuracy, particularly when it is intended for a fragile population such as newborns, subject to a high risk of therapeutic failure or toxicity.
Furthermore, experimental tests modelled in fluid dynamic studies must always be validated with clinical evaluations.
Further studies are needed to validate the FDA’s statement about improved accuracy with the use of bulk bottle adapters, a condition that could not be assessed in this study.
The ENFitTM LDT should therefore be used with great caution in newborns until new data are available demonstrating the reliability of this syringe.
In all clinical and healthcare settings, it is essential to carefully consider the specific needs of patients. The ENFitTM connector has been designed to offer increased safety in feeding and drug administration procedures, and is the appropriate choice for both the adult and paediatric population.
In the neonatal patient, and even more so in preterm infants, special needs must be taken into account in addition to safety. Getting educated and informed through clinical guidelines, clinical recommendations and scientific studies remains the best way to ensure we make the most appropriate choice for these fragile patients. Only through the use of devices appropriate to the size and needs of this specific population can we ensure maximum safety and care for newborns.
The main priority must always be the safety and well-being of patients.
Bibliography
- ISO 80369-1: Small-bore connectors for liquids and gases in health-care applications. International Organization for Standardization. https://www.iso.org/obp/ ui/#iso:std:45976:en. Published December 2010. Accessed January 31, 2022.
- ISO 80369-3: Small-bore connectors for liquids and gases in health-care applications – Part 3: Connectors for enteral applications. International Organization for Standardization. https://www.iso.org/obp/ui/#iso:std:50731:en. Published July 2017. Accessed January 31, 2022.
- Institute for Safe Medication Practices. ENFit enteral devices are on their way…Important safety considerations for hospitals. ISMP Medication Safety Alert. https://www.ismp.org/resources/enfit-enteral-devices-are-their-wayimportant-safety-considerations-hospitals. . Published April 9, 2015. Accessed January 31, 2022.
- ISO 20695: Enteral feeding systems-Design and testing.International Organization for Standardization. https:// www.iso.org/standard/68853.html. Published March 2020. Accessed January 31, 2022.
- O’Mara K, Gattoline SJ, Campbell CT. Female low dose tip syringes-increased complexity of use may compromise dosing accuracy in patients. J Clin Pharm Ther. 2019;44(3):463-470.
- O’Mara K, Campbell C. Dosing inaccuracy with enteral use of ENFitR low-dose tip syringes: The risk beyond oral adapters. J Clin Pharm Ther. 2020;52(2):335-339.
- Guha S, Niswander W, Herman A, Antonino MJ, et al. Assessing dosing errors in legacy and low dose tip ENFitR syringes. J Clin Pharm Ther. 2022;47(2):218-227.
- O’Mara, Keliana, Christopher Campbell, and Ryan O’Mara. “Comparison of Dosing Accuracy Between the ENFit LDT and a Neonatal-Specific ISO-Compliant Enteral Syringe.” The Journal of Pediatric Pharmacology and Therapeutics 28.3 (2023): 255-261.





